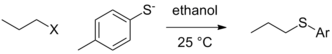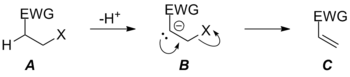Leaving group
[1] However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage.
Nevertheless, one can generally examine acid dissociation constants to qualitatively predict or rationalize rate or reactivity trends relating to variation of the leaving group.
Consistent with this picture, strong bases such as OH−, OR2 and NR−2 tend to make poor leaving groups, due their inability to stabilize a negative charge.
Substrates containing phosphate and carboxylate leaving groups are more likely to react by competitive addition-elimination, while sulfonium and ammonium salts generally form ylides or undergo E2 elimination when possible.
Hydroxide, alkoxides, amides, hydride, and alkyl anions do not serve as leaving groups in SN2 reactions.
On the other hand, when anionic or dianionic tetrahedral intermediates collapse, the high electron density of the neighboring heteroatom facilitates the expulsion of a leaving group.
For example, in SNAr reactions, the rate is generally increased when the leaving group is fluoride relative to the other halogens.
This effect is due to the fact that the highest energy transition state for this two step addition-elimination process occurs in the first step, where fluoride's greater electron withdrawing capability relative to the other halides stabilizes the developing negative charge on the aromatic ring.
In Friedel-Crafts alkylations, the normal halogen leaving group order is reversed so that the rate of the reaction follows RF > RCl > RBr > RI.
[7] It is common in E1 and SN1 reactions for a poor leaving group to be transformed into a good one by protonation or complexation with a Lewis acid.
In the vast majority of cases, reactions that involve leaving group activation generate a cation in a separate step, before either nucleophilic attack or elimination.
Under forcing conditions, even amides can be made to undergo basic hydrolysis, a process that involves the expulsion of an extremely poor leaving group, R2N−.
This reaction is facilitated by the fact that the leaving group is most likely an arylcopper compound rather than the much more basic alkali metal salt.
Because only one transition state connects starting material A and product C, the reaction is now concerted (albeit very asynchronous in the pictured case) due to the increase in leaving group ability of X.
Compounds where loss of a super leaving group can generate a stable carbocation are usually highly reactive and unstable.
Part of the exceptional reactivity of compounds of hyper leaving groups has been ascribed to the entropic favorability of having one molecule split into three.
Heating neat samples of (CH3)2Cl+ [CHB11Cl11]− under reduced pressure resulted in methylation of the very poorly nucleophilic carborane anion with concomitant expulsion of the CH3Cl leaving group.