Hydroxyproline
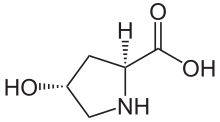
[1] Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom.
Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl hydroxylase following protein synthesis (as a post-translational modification).
Under normoxia (normal oxygen conditions) EGLN1[1] protein hydroxylates the proline at the 564 position of HIF-1 alpha, which allows ubiquitylation by the von Hippel-Lindau tumor suppressor (pVHL) and subsequent targeting for proteasome degradation.
[9] These hydroxyprolines serve as the attachment points for glycan chains which are added as post-translational modifications.
The most obvious, first effects (gingival and hair problems) of absence of ascorbic acid in humans come from the resulting defect in hydroxylation of proline residues of collagen, with reduced stability of the collagen molecule, causing scurvy.
The most notable ones are 2,3-cis-, 3,4-trans-, and 3,4-dihydroxyproline, which occurs in diatom cell walls[12] and are postulated to have a role in silica deposition.