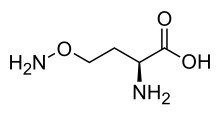
Canaline
l-Canaline is the only naturally occurring amino acid known that has an O-alkyl hydroxylamine functionality in the side chain.
Its toxicity stems primarily from the fact that it readily forms oximes with keto acids and aldehydes, especially the pyridoxal phosphate cofactor of many vitamin B6-dependent enzymes.
l-Canaline is a substrate for ornithine aminotransferase resulting in the synthesis of l-ureidohomoserine (the corresponding analog of l-citrulline).
Every time a canavanine molecule runs through the canaline-urea cycle, the two terminal nitrogen atoms are released as urea.
Urea is an important by-product of this reaction sequence because it makes ammonia (urease-mediated) that is available to support intermediary nitrogen metabolism.