Tonicity
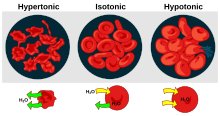
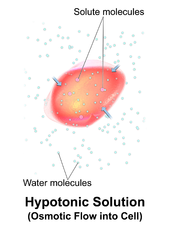
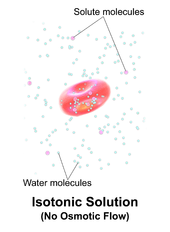
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane.
Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux.
It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution.
The cells often take on the appearance of a pincushion, and the plasmodesmata almost cease to function because they become constricted, a condition known as plasmolysis.
They respond to the loss by drinking large amounts of saltwater, and actively excreting the excess salt.
For example, an iso-osmolar urea solution is hypotonic to red blood cells, causing their lysis.
Neither sodium nor chloride ions can freely pass through the plasma membrane, unlike urea.