Two-dimensional nuclear magnetic resonance spectroscopy
[1] In 2D NMR, signals are distributed across two frequency axes, providing improved resolution and separation of overlapping peaks, particularly beneficial for studying complex molecules.
This technique identifies correlations between different nuclei within a molecule, facilitating the determination of connectivity, spatial proximity, and dynamic interactions.
These techniques are indispensable in fields such as structural biology, where they are pivotal in determining protein and nucleic acid structures; organic chemistry, where they aid in elucidating complex organic molecules; and materials science, where they offer insights into molecular interactions in polymers and metal-organic frameworks.
The first two-dimensional experiment, COSY, was proposed by Jean Jeener, a professor at the Université Libre de Bruxelles, in 1971.
This experiment was later implemented by Walter P. Aue, Enrico Bartholdi and Richard R. Ernst, who published their work in 1976.
The first and most popular two-dimension NMR experiment is the homonuclear correlation spectroscopy (COSY) sequence, which is used to identify spins which are coupled to each other.
By detecting these interactions, COSY provides vital information about the connectivity between atoms within a molecule, making it a crucial tool for structural elucidation in organic chemistry.
The COSY experiment generates a two-dimensional spectrum with chemical shifts along the x-axis (horizontal) and y-axis (vertical) and involves several key steps.
[1] First, the sample is excited using a series of radiofrequency (RF) pulses, bringing the nuclear spins into a higher energy state.
After the first RF pulse, the system evolves freely for a period called t1, during which the spins precess at frequencies corresponding to their chemical shifts.
This series of experiments, each with a different value of t1, allows for the detection of chemical shifts from nuclei that may not be observed directly in a one-dimensional spectrum.
As t1 is incremented, cross-peaks are produced in the resulting 2D spectrum, representing interactions like coupling or spatial proximity between nuclei.
This approach helps map out atomic connections, providing deeper insight into molecular structure and aiding in the interpretation of complex systems.
The data are then processed through Fourier transformation along both the t1 and t2 axes, creating a 2D spectrum with peaks plotted along the diagonal and off-diagonal.
The key feature of a COSY spectrum is the presence of cross-peaks as shown in Figure 1, indicating coupling between pairs of nuclei.
COSY is especially useful when dealing with complex molecules such as natural products, peptides, and proteins, where understanding the connectivity of different nuclei through bonds is crucial.
The two-dimensional spectrum that results from the COSY experiment shows the frequencies for a single isotope, most commonly hydrogen (1H) along both axes.
Recent advances in this technique include the 1D-CSSF (chemical shift selective filter) TOCSY experiment, which produces higher quality spectra and allows coupling constants to be reliably extracted and used to help determine stereochemistry.
With the advent of techniques for suppressing these undesired signals, inverse correlation experiments such as HSQC, HMQC, and HMBC are actually much more common today.
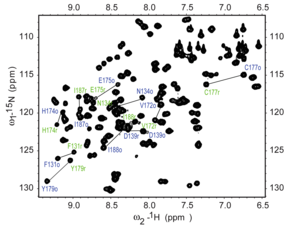
[15] Heteronuclear Single Quantum Coherence (HSQC) is a 2D NMR technique utilized for the detection of interactions between different types of nuclei which are separated by one bond, particularly a proton (1H) and a heteronucleus such as carbon (13C) or nitrogen (15N).
[18] This method plays a role in structural elucidation, particularly in analyzing organic compounds, natural products, and biomolecules such as proteins and nucleic acids.
An extra spin echo step can then optionally be used to decouple the signal, simplifying the spectrum by collapsing multiplets to a single peak.
[18] Interpretation of the HSQC spectrum is based on the observation of cross-peaks, which indicates the direct bonding between protons and carbons or nitrogens.
Each cross-peak corresponds to a specific 1H-13C or 1H-15N pair, providing direct assignments of 1H-Xconnectivity, where X is the heteronucleus[17] The HSQC technique offers several advantages, including its focus on one-bond correlations, increased sensitivity due to the direct detection of protons, and the simplification of crowded spectra by resolving overlapping signals and aiding in the analysis of complex molecules.
[20] One application of NOESY is in the study of large biomolecules, such as in protein NMR, in which relationships can often be assigned using sequential walking.
This only reveals which peaks have measurable NOEs to the resonance of interest but takes much less time than the full 2D experiment.
In addition, if a pre-selected nucleus changes environment within the time scale of the experiment, multiple negative signals may be observed.