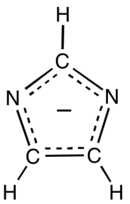
Imidazolate
It is a nucleophile and a strong base.
The free anion has C2v symmetry.
Imidazole has a pKa of 14.05,[1] so the deprotonation of imidazole (C3H3N2H) requires a strong base.
Imidazolate is a common bridging ligand in coordination chemistry.
[4][5] In the enzyme superoxide dismutase, imidazolate links copper and zinc centers.