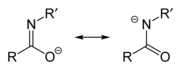
Carboximidate
[1] Imidates may be generated by a number of synthetic routes,[2] but are in general formed by the Pinner reaction.
[2] They can be hydrolyzed to give esters and by an analogous process react with amines (including ammonia) to form amidines.
[4] It is named after Arthur William Chapman, who first described it,[5] and is conceptually similar to the Newman–Kwart rearrangement.
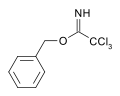
[6] For example, the base catalyzed reaction of benzyl alcohol upon trichloroacetonitrile yields a trichloroacetimidate.
This species has orthogonal stability to acetate and TBS protections and may be cleaved by acid hydrolysis.