Chemical impurity
In chemistry and materials science, impurities are chemical substances inside a confined amount of liquid, gas, or solid.
Standards have been established by various organizations that attempt to define the permitted levels of various impurities in a manufactured product.
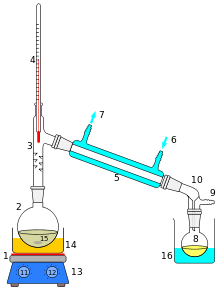
The removal of unwanted impurities may require the use of separation or purification techniques such as distillation or zone refining.
In other cases, impurities might be added to acquire certain properties of a material such as the color in gemstones or conductivity in semiconductors.
Examples include ash and debris in metals and leaf pieces in blank white papers.
[4] Impurities in pharmaceuticals and therapeutics are of special concern and the last couple of decades have witnessed a fair number of scandals, from insecure ingredients and incorrect dosage forms to intentionally fortified medications and accidental contaminations.
These impurities can be naturally occurring and left unaltered in a material or be intentionally added during synthesis.
These types of impurities can show up in our day-to-day lives such as different colors in gemstones or by doping to tune the conductivity of semiconductors.
Pure beryl will appear colorless but this rarely occurs and the presence of trace elements change its color.
The dopants, the elements added to the original crystal structure, contain a different number of electrons then the base formula.
For example, the presence of foreign elements may have important effects on the mechanical and magnetic properties of metal alloys.