In vitro maturation
In 1935, Pincus & Enzmann did the first experiment on immature rabbit oocyte, showing in vitro spontaneous maturation and fertilization.
[1] In 1965 Edwards then continued IVM studies in mouse, sheep, cow, pig, rhesus monkey and human.
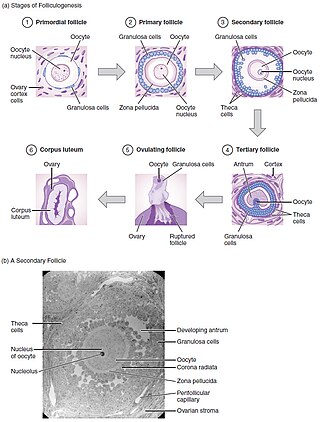
[5] Oogenesis takes place during fetal life, in which primordial germ cells undergo mitosis until a few weeks prior to birth, forming oogonia.
This results in an expanding or dispersed pattern of the cumulus oophorus around the egg cell, facilitating its identification within follicular fluid.
[16] A few live births have already been made by taking small early tertiary follicles, letting them mature in vitro and subsequently fertilizing them.
[17] In vitro maturation is an assistive reproductive technique (ART) typically used in patients with fertility issues including polycystic ovary syndrome (PCOS), high antral follicle counts and ovarian hyper-responsiveness.
[18][19] However, more recently IVM has also become widely utilised in areas such as fertility preservation in cancer patient who have undergone treatment involving gonadotoxic therapies.
PCOS involves discrepancies in the Hyphophyseal-pituitary-gonadal endocrine axis which can result in hormonal dysfunction, excess androgens (e.g. testosterone) and frequent anovulatory menstrual cycles.
[20][21] Few studies shows that substituting IVM in PCOS patients eliminates the risk of OHSS and lowers the cost of treatment.
[19][21] In IVM, immature oocytes are removed from the antral follicle of a woman and then are matured in vitro in a culture rich in gonadotrophins.
There is also research required into whether or not babies born to mothers who have undergone IVM have any health concerns (e.g. developmental issues) later in life.
[19] Ovarian tissue cryopreservation can be used as a method of fertility preservation, such as before undergoing chemotherapy that can cause female infertility, or as a future resource in case the oocytes will stop functioning by advanced maternal age.
However, rescue IVM has been considered a controversial field: If oocytes have not matured sufficiently in vivo – despite exposure to significant levels of gonadotrophins – it may be indicative of dysmaturity and of a limited potential developmentally.
[19] IVM has also been used in domestic animals including mice,[24] cats,[25][26] dogs,[27][28] swine,[29] sheep,[30] horse[31] and cattle[32][33] as well as wild species such as buffalo,[34] bison,[35] fish,[36] lions,[37] tigers[37] and leopards.
[37] The ability to recover animals' oocytes initially destined for ovarian follicle atresia, can be utilized by researchers, conservationists and the agriculture industry for academic purposes or for improving breeding systems.
Animal IVM models can be used to study how different exposures to harmful and toxic substances affect the maturing oocytes and their ability to become fertilized and develop into an embryo.
[39] It can also be used for subsequent biotechnology applications such as for the creation of transgenic animals using innovative gene-editing techniques such as CRISPR/Cas9, TALENs and ZFNs for biomedical research.
In agriculture, IVM is usually carried out prior to IVF or artificial insemination as a means of conserving desirable traits of particular animals within herds and counteracting lower production as a result of seasonal breeding.
In livestock species such cattle, transvaginal oocyte recovery from the ovaries of live female animals can be repeatedly carried out prior to the in vitro production of embryos.
[42] However, due to limited resources and the species-specific nature of assisted reproductive technologies, the application of techniques such as IVM is still rare for non-domesticated animals.
[42] In an experiment by Segers I et al. (2015), the overall maturation rate after IVM of oocytes recovered from ovariectomy specimens in laboratory was 36%.
This experiment shows that IVM of oocytes obtained ex vivo during the processing of ovarian cortex prior to cryopreservation is a promising solution for patients at risk for fertility loss.
[43] The success of embryo production in vitro depends upon the use of an efficient oocyte retrieval technique and the best results have been obtained by laparoscopic aspiration.
However, no data to date has proven this benefit in fetal development and birth of healthy offspring after embryo transfer to surrogate females.
[49] In a research by Wang X et al. (2014), gonadotropins affect oocyte maturation, fertilisation and developmental competence in vitro.