Indium gallium arsenide
The principal importance of GaInAs is its application as a high-speed, high sensitivity photodetector of choice for optical fiber telecommunications.
By far, the most important alloy composition from technological and commercial standpoints is Ga0.47In0.53As, which can be deposited in single crystal form on indium phosphide (InP).
With the addition of 1.5% InAs to the alloy, In0.015Ga0.985As becomes latticed-matched to the Ge substrate, reducing stress in subsequent deposition of GaAs.
A good match between the lattice constants of the film and substrate is required to maintain single crystal properties and this limitation permits small variations in composition on the order of a few percent.
Therefore, the properties of epitaxial films of GaInAs alloys grown on GaAs are very similar to GaAs and those grown on InAs are very similar to InAs, because lattice mismatch strain does not generally permit significant deviation of the composition from the pure binary substrate.
The bandgap energy of GaInAs can be determined from the peak in the photoluminescence spectrum, provided that the total impurity and defect concentration is less than 5×1016 cm−3.
The bandgap energy at room temperature for standard InGaAs/InP (53% InAs, 47% GaAs), is 0.75 eV and lies between that of Ge and Si.
By coincidence the bandgap of GaInAs is perfectly placed for photodetector and laser applications for the long-wavelength transmission window, (the C-band and L-band) for fiber-optic communications.
The electron effective mass of GaInAs m*/m° = 0.041 [10] is the smallest for any semiconductor material with an energy bandgap greater than 0.5 eV.
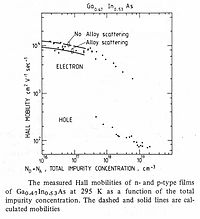
In practical terms, a low effective mass leads directly to high carrier mobility, favoring higher speed of transport and current carrying capacity.
A lower carrier effective mass also favors increased tunneling current, a direct result of delocalization.
The room temperature diffusion constant for electrons at 250 cm2·s−1 is significantly larger than that of Si, GaAs, Ge or InP, and determines the ultra-fast response of Ga0.47In0.53As photodetectors.
These devices are especially useful for detection of single photons in applications such as quantum key distribution where response time is not critical.
Avalanche photodetectors require a special structure to reduce reverse leakage current due to tunnelling.
Other important innovations include the integrated photodiode – FET receiver[23] and the engineering of GaInAs focal-plane arrays.
[25] GaInAs/InAlAs quantum-well lasers can be tuned to operate at the λ = 1500 nm low-loss, low-dispersion window for optical fiber telecommunications [26] In 1994, GaInAs/AlInAs quantum wells were used by Jérôme Faist and co-workers[27] who invented and demonstrated a new kind of semiconductor laser based on photon emission by an electron making an optical transition between subbands in the quantum well.
This is a promising accomplishment, but more work is needed to show that the reduced size results in improved electronic performance relative to that of silicon or GaAs-based transistors.
In 2014, Researchers at Penn State University developed a novel device prototype designed to test nanowires made of compound semiconductors such as InGaAs.
Inhalation of these gases neutralizes oxygen absorption by the bloodstream and can be fatal within a few minutes if toxic dose levels are exceeded.
Safe handling involves using a sensitive toxic gas detection system and self-contained breathing apparatus.
[34] Once GaInAs is deposited as a thin film on a substrate, it is basically inert and is resistant to abrasion, sublimation or dissolution by common solvents such as water, alcohols or acetones.
The National Institutes of Health studied these materials and found:[35] The World Health Organization's International Agency for Research on Cancer's review of the NIH toxicology study concluded:[36] REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) is a European initiative to classify and regulate materials that are used, or produced (even as waste) in manufacturing.
The principal exposure risk occurs during substrate preparation where grinding and polishing generate micron-size particles of GaAs and InP.