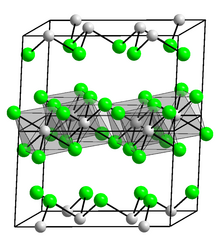
Indium(III) chloride
Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate.
This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid.
This unstable compound decomposes below 0 °C,[8] and is reacted in situ in organic synthesis as a reducing agent[9] and to prepare tertiary amine and phosphine complexes of InH3.
As an example of the latter,[12] the reaction proceeds at room temperature, with 1 mole% catalyst loading in an acetonitrile-water solvent mixture.
The first step is a Knoevenagel condensation between the barbituric acid and the aldehyde; the second step is a reverse electron-demand Diels-Alder reaction, which is a multicomponent reaction of N,N'-dimethyl-barbituric acid, benzaldehyde and ethyl vinyl ether.