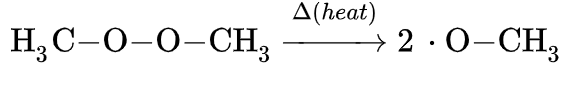Initiation (chemistry)
Initiation creates a reactive centre on a molecule which produces a chain reaction.
This process can produce very long chains of molecules called polymers, which are the building blocks for many materials.
[4] Copolymerization, which is when different kinds of monomers are initiated and react with each other, is more stable and can happen at lower temperatures than Homopolymerization.
[4] Self-initiation between homo-monomers is a difficult mechanism to observe because species that are initiated aren't always the same kind of monomer.
LED light passes through the reaction flask which excites the monomers turning them into reactive species, mainly radicals and ions, which can then polymerize.