Insertion reaction
[2] When these reactions are reversible, the removal of the small molecule from the metal-ligand bond is called extrusion or elimination.
Organic Syntheses provides the example of t-BOC protected (S)-phenylalanine (2-amino-3-phenylpropanoic acid) being reacted sequentially with triethylamine, ethyl chloroformate, and diazomethane to produce the α-diazoketone, which is then reacted with silver trifluoroacetate / triethylamine in aqueous solution to generate the t-BOC protected form of (S)-3-amino-4-phenylbutanoic acid.
[6] A related transformation is the Nierenstein reaction in which a diazomethane methylene group is inserted into the carbon-chlorine bond of an acid chloride to generate an α-chloro ketone.
When tosyl azide reacts with norbornadiene, a ring expansion reaction takes place in which a nitrogen atom is inserted into a carbon-carbon bond α- to the bridge head:[14] The Beckmann rearrangement[15][16] is another example of a ring expanding reaction in which a heteroatom is inserted into a carbon-carbon bond.
The most important application of this reaction is the conversion of cyclohexanone to its oxime, which is then rearranged under acidic conditions to provide ε-caprolactam,[17] the feedstock for the manufacture of Nylon 6.
The insertion of carbon monoxide and alkenes into metal-carbon bonds is a widely exploited reaction with major industrial applications.
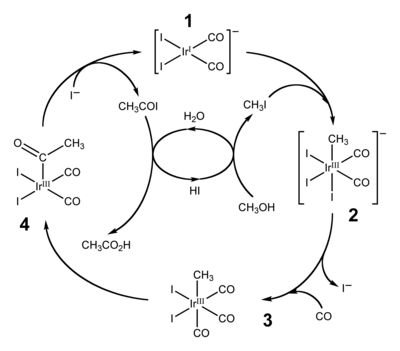
[23][24] By 2002, worldwide annual production of acetic acid stood at 6 million tons, of which approximately 60% is produced by the Cativa process.
The active catalyst species is regenerated by the reductive elimination of acetyl iodide from (4), a de-insertion reaction.
[23] The insertion of ethylene and propylene into titanium alkyls is the cornerstone of Ziegler-Natta catalysis, the commercial route of polyethylene and polypropylene.
This technology mainly involves heterogeneous catalysts, but it is widely assumed that the principles and observations on homogeneous systems are applicable to the solid-state versions.