Sulfur dioxide
It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur-bearing fossil fuels.
[9] Sulfur dioxide is somewhat toxic to humans, although only when inhaled in relatively large quantities for a period of several minutes or more.
[13] It has been implicated as a key agent in the warming of early Mars, with estimates of concentrations in the lower atmosphere as high as 100 ppm,[14] though it only exists in trace amounts.
The James Webb Space Telescope has observed the presence of sulfur dioxide on the exoplanet WASP-39b, where it is formed through photochemistry in the planet's atmosphere.
Many different bonding modes (geometries) are recognized, but in most cases, the ligand is monodentate, attached to the metal through sulfur, which can be either planar and pyramidal η1.
However, such solutions do show spectra of the hydrogen sulfite ion, HSO3−, by reaction with water, and it is in fact the actual reducing agent present: In the beginning of the 20th century sulfur dioxide was used in Buenos Aires as a fumigant to kill rats that carried the Yersinia pestis bacterium, which causes bubonic plague.
In Buenos Aires, where these apparatuses were known as Sulfurozador, but later also in Rio de Janeiro, New Orleans and San Francisco, the sulfur dioxide treatment machines were brought into the streets to enable extensive disinfection campaigns, with effective results.
[33] Sulfur dioxide or its conjugate base bisulfite is produced biologically as an intermediate in both sulfate-reducing organisms and in sulfur-oxidizing bacteria, as well.
It is considered that endogenous sulfur dioxide plays a significant physiological role in regulating cardiac and blood vessel function, and aberrant or deficient sulfur dioxide metabolism can contribute to several different cardiovascular diseases, such as arterial hypertension, atherosclerosis, pulmonary arterial hypertension, and stenocardia.
Authors considered homocysteine to be one of useful biochemical markers of disease severity and sulfur dioxide metabolism to be one of potential therapeutic targets in those patients.
Treatment of aryl diazonium salts with sulfur dioxide and cuprous chloride yields the corresponding aryl sulfonyl chloride, for example:[40] As a result of its very low Lewis basicity, it is often used as a low-temperature solvent/diluent for superacids like magic acid (FSO3H/SbF5), allowing for highly reactive species like tert-butyl cation to be observed spectroscopically at low temperature (though tertiary carbocations do react with SO2 above about −30 °C, and even less reactive solvents like SO2ClF must be used at these higher temperatures).
[41] Being easily condensed and possessing a high heat of evaporation, sulfur dioxide is a candidate material for refrigerants.
Sulfur dioxide content in naturally-released geothermal gasses is measured by the Icelandic Meteorological Office as an indicator of possible volcanic activity.
[42] In the United States, the Center for Science in the Public Interest lists the two food preservatives, sulfur dioxide and sodium bisulfite, as being safe for human consumption except for certain asthmatic individuals who may be sensitive to them, especially in large amounts.
[43] Symptoms of sensitivity to sulfiting agents, including sulfur dioxide, manifest as potentially life-threatening trouble breathing within minutes of ingestion.
[44] Sulphites may also cause symptoms in non-asthmatic individuals, namely dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea, and even life-threatening anaphylaxis.
[47] In 2008, the American Conference of Governmental Industrial Hygienists reduced the short-term exposure limit to 0.25 parts per million (ppm).
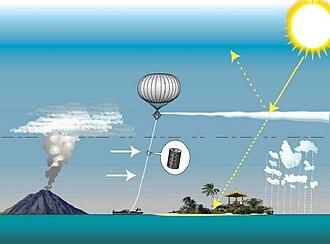
"[47] Major volcanic eruptions have an overwhelming effect on sulfate aerosol concentrations in the years when they occur: eruptions ranking 4 or greater on the Volcanic Explosivity Index inject SO2 and water vapor directly into the stratosphere, where they react to create sulfate aerosol plumes.
[49] Volcanic emissions vary significantly in composition, and have complex chemistry due to the presence of ash particulates and a wide variety of other elements in the plume.
[50] However, before the Industrial Revolution, dimethyl sulfide pathway was the largest contributor to sulfate aerosol concentrations in a more average year with no major volcanic activity.
At the time, the most visible one was acid rain, caused by precipitation from clouds carrying high concentrations of sulfate aerosols in the troposphere.
[55] At its peak, acid rain has eliminated brook trout and some other fish species and insect life from lakes and streams in geographically sensitive areas, such as Adirondack Mountains in the United States.
[56] Acid rain worsens soil function as some of its microbiota is lost and heavy metals like aluminium are mobilized (spread more easily) while essential nutrients and minerals such as magnesium can leach away because of the same.
Ultimately, plants unable to tolerate lowered pH are killed, with montane forests being some of the worst-affected ecosystems due to their regular exposure to sulfate-carrying fog at high altitudes.
[57][58][59][60][61] While acid rain was too dilute to affect human health directly, breathing smog or even any air with elevated sulfate concentrations is known to contribute to heart and lung conditions, including asthma and bronchitis.
This improvement resulted in part from flue-gas desulfurization, a technology that enables SO2 to be chemically bound in power plants burning sulfur-containing coal or petroleum.
To control sulfur emissions, dozens of methods with relatively high efficiencies have been developed for fitting of coal-fired power plants.
[68] Sulfur dioxide aerosols in the stratosphere can contribute to ozone depletion in the presence of chlorofluorocarbons and other halogenated ozone-depleting substances.
[69] Injection of sulfur dioxide and large amounts of water vapor into the stratosphere following the 2022 eruption of Hunga Tonga-Hunga Haʻapai resulted in altered atmospheric circulation that promoted a decrease in ozone in the southern latitudes but an increase in the tropics.
In fact, it is impossible to fully estimate the warming impact of all greenhouse gases without accounting for the counteracting cooling from aerosols.