Insulin
[10] Decreased or absent insulin activity results in diabetes, a condition of high blood sugar level (hyperglycaemia).
In type 1 diabetes, the beta cells are destroyed by an autoimmune reaction so that insulin can no longer be synthesized or be secreted into the blood.
[34] An increase in blood glucose levels causes phosphorylation of PDX1, which leads it to undergo nuclear translocation and bind the A3 element within the insulin promoter.
Increased blood glucose can after a while destroy the binding capacities of these proteins, and therefore reduce the amount of insulin secreted, causing diabetes.
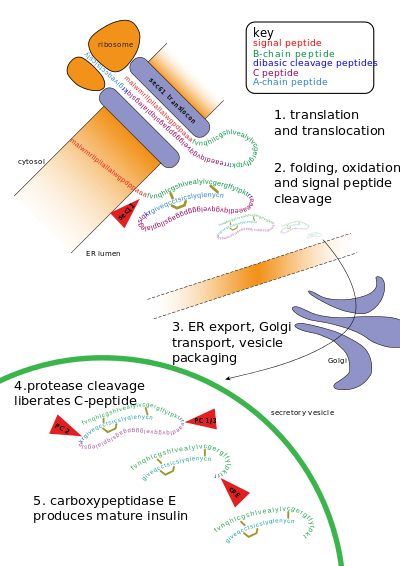
The second phase is a sustained, slow release of newly formed vesicles triggered independently of sugar, peaking in 2 to 3 hours.
[50] Reduced first-phase insulin release may be the earliest detectable beta cell defect predicting onset of type 2 diabetes.
[59] The net effect of norepinephrine from sympathetic nerves and epinephrine from adrenal glands on insulin release is inhibition due to dominance of the α-adrenergic receptors.
[61] This may be achieved by delivering insulin rhythmically to the portal vein, by light activated delivery, or by islet cell transplantation to the liver.
This activity provokes the autophosphorylation of the β subunits and subsequently the phosphorylation of proteins inside the cell known as insulin receptor substrates (IRS).
[66] The cascade that leads to the insertion of GLUT4 glucose transporters into the cell membranes of muscle and fat cells, and to the synthesis of glycogen in liver and muscle tissue, as well as the conversion of glucose into triglycerides in liver, adipose, and lactating mammary gland tissue, operates via the activation, by IRS-1, of phosphoinositol 3 kinase (PI3K).
[88][89] This dysregulation contributes to excessive visceral fat accumulation and reduced adiponectin release from abdominal adipose tissue, and further to the onset of several cardiometabolic risk factors that are associated with obesity and type 2 diabetes.
[91] This may result in a variety of symptoms including clumsiness, trouble talking, confusion, loss of consciousness, seizures or death.
[103][104] Insulin is also used in many cell lines, such as CHO-s, HEK 293 or Sf9, for the manufacturing of monoclonal antibodies, virus vaccines, and gene therapy products.
Featuring extra-thin walls and a multi-bevel tapered point, these pen needles prioritise patient comfort by minimising pain and ensuring seamless medication delivery.
[109] Insulin, and all other medications, are supplied free of charge to people with diabetes by the National Health Service in the countries of the United Kingdom.
[111] The function of the "little heaps of cells", later known as the islets of Langerhans, initially remained unknown, but Édouard Laguesse later suggested they might produce secretions that play a regulatory role in digestion.
Between 1911 and 1912, E.L. Scott at the University of Chicago tried aqueous pancreatic extracts and noted "a slight diminution of glycosuria", but was unable to convince his director of his work's value; it was shut down.
[116] In 1916, Nicolae Paulescu developed an aqueous pancreatic extract which, when injected into a diabetic dog, had a normalizing effect on blood sugar levels.
A surgeon by training, Banting knew that blockages of the pancreatic duct would lead most of the pancreas to atrophy, while leaving the islets of Langerhans intact.
This proved unfortunate for Noble, as Banting kept Best for the entire summer and eventually shared half his Nobel Prize money and credit for the discovery with Best.
Over the spring of 1922, Best managed to improve his techniques to the point where large quantities of insulin could be extracted on demand, but the preparation remained impure.
In November, Lilly's head chemist, George B. Walden discovered isoelectric precipitation and was able to produce large quantities of highly refined insulin.
Toward the end of January 1922, tensions mounted between the four "co-discoverers" of insulin and Collip briefly threatened to separately patent his purification process.
However, concerns remained that a private third-party would hijack and monopolize the research (as Eli Lilly and Company had hinted[134]), and that safe distribution would be difficult to guarantee without capacity for quality control.
He isolated and purified total messenger RNA from pancreatic islet cells which was then translated in oocytes from Xenopus laevis and precipitated using anti-insulin antibodies.
When total translated protein was run on an SDS-polyacrylamide gel electrophoresis and sucrose gradient, peaks corresponding to insulin and proinsulin were isolated.
Following this oral presentation, Weber was invited to dinner to discuss his paper and findings by Donald Steiner, a researcher who contributed to the characterization of proinsulin.
[14][15] Genentech, founded by Swanson, Boyer and Eli Lilly and Company, went on in 1982 to sell the first commercially available biosynthetic human insulin under the brand name Humulin.
[16] Recently, another recombinant approach has been used by a pioneering group of Canadian researchers, using an easily grown safflower plant, for the production of much cheaper insulin.
[151] The work published by Banting, Best, Collip and Macleod represented the preparation of purified insulin extract suitable for use on human patients.