Intermediate filament
Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates.
[4] Intermediate filaments are composed of a family of related proteins sharing common structural and sequence features.
[1][5] Animal intermediate filaments are subcategorized into six types based on similarities in amino acid sequence and protein structure.
[7] The structure of proteins that form intermediate filaments (IF) was first predicted by computerized analysis of the amino acid sequence of a human epidermal keratin derived from cloned cDNAs.
[9][10] The central building block of an intermediate filament is a pair of two intertwined proteins that is called a coiled-coil structure.
Structural analysis of a pair of keratins shows that the two proteins that form the coiled-coil bind by hydrophobic interactions.
[13] Part of the assembly process includes a compaction step, in which ULF tighten and assume a smaller diameter.
[17] C-terminal "tail domain" shows extreme length variation between different IF proteins.
Also, unlike actin or tubulin, intermediate filaments do not contain a binding site for a nucleoside triphosphate.
However, different kinds of IFs share basic characteristics: In general, they are all polymers that measure between 9–11 nm in diameter when fully assembled.
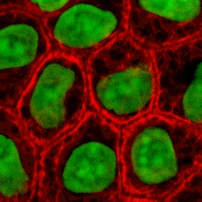
[6] Cytokeratin filaments laterally associate with each other to create a thick bundle of ~50 nm radius.
[21] Subsequently, these bundles would intersect through junctions to form a dynamic network, spanning the cytoplasm of epithelial cells.
In addition, a few other diverse types of eukaryotes have lamins, suggesting an early origin of the protein.