Internal pressure
It has the same dimensions as pressure, the SI unit of which is the pascal.
It is defined as a partial derivative of internal energy with respect to volume at constant temperature: Internal pressure can be expressed in terms of temperature, pressure and their mutual dependence: This equation is one of the simplest thermodynamic equations.
More precisely, it is a thermodynamic property relation, since it holds true for any system and connects the equation of state to one or more thermodynamic energy properties.
at constant temperature gives: And using one of the Maxwell relations: In a perfect gas, there are no potential energy interactions between the particles, so any change in the internal energy of the gas is directly proportional to the change in the kinetic energy of its constituent species and therefore also to the change in temperature: The internal pressure is taken to be at constant temperature, therefore i.e. the internal energy of a perfect gas is independent of the volume it occupies.
It follows directly from the thermodynamic equation of state if we use the ideal gas law
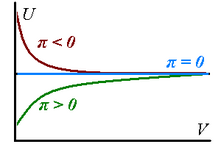
We have Real gases have non-zero internal pressures because their internal energy changes as the gases expand isothermally - it can increase on expansion (
, signifying presence of dominant attractive forces between the particles of the gas) or decrease (
In the limit of infinite volume these internal pressures reach the value of zero: corresponding to the fact that all real gases can be approximated to be perfect in the limit of a suitably large volume.
parameter models the effect of attractive forces between molecules in the gas.
However, real non-ideal gases may be expected to exhibit a sign change between positive and negative internal pressures under the right environmental conditions if repulsive interactions become important, depending on the system of interest.
Loosely speaking, this would tend to happen under conditions of temperature and pressure such that
In addition, through the use of the Euler chain relation it can be shown that Defining
This coefficient is often small, and usually negative at modest pressures (as predicted by the van der Waals equation).
James Joule tried to measure the internal pressure of air in his expansion experiment by adiabatically pumping high pressure air from one metal vessel into another evacuated one.
The actual deviations from the perfect behaviour were not observed since they are very small and the specific heat capacity of water is relatively high.
Much later, in 1925 Frederick Keyes and Francis Sears published measurements of the Joule effect for carbon dioxide at
Under these conditions the temperature dropped when the pressure was adiabatically lowered, which indicates that
This is consistent with the van der Waals gas prediction that