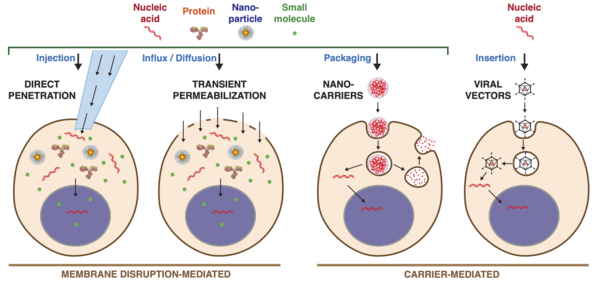
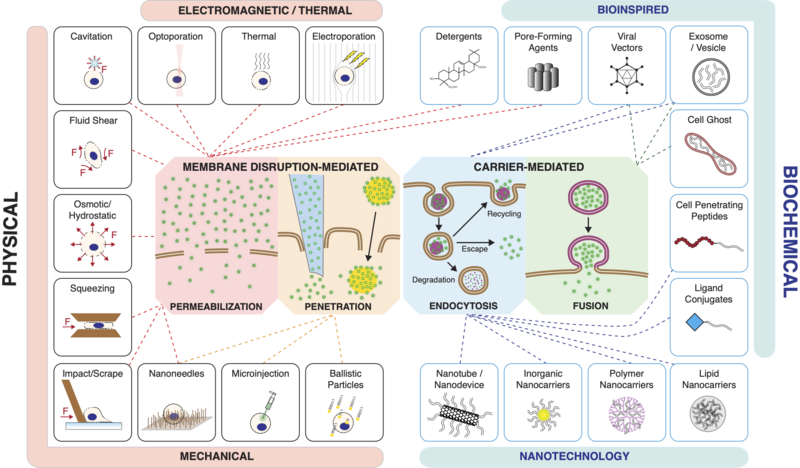
Intracellular delivery
Materials that are delivered into cells include nucleic acids (DNA and RNA), proteins, peptides, impermeable small molecules, synthetic nanomaterials, organelles, and micron-scale tracers, devices and objects.
Medical applications of intracellular delivery range from in vitro fertilisation (IVF)[3] and mRNA vaccines[4] to gene therapy[5] and preparation of CAR-T cells.
[9] Intracellular delivery is a fundamental technique in the study of biology and genetics, such as the use of DNA plasmid transfection to investigate protein function in living cells.
Plasmid DNA began to be transfected into animal cells for the purpose of gene expression in the late 1970s via microinjection [18] and calcium phosphate methods.
[23] Since their Nobel prize winning discovery in 1998, siRNA have been transfected into cells in thousands of biological studies in order to perturb gene function.
Because proteins have diverse size, shape and charge, they cannot easily be delivered into cells with one-size-fits-all solutions that cationic lipids use for nucleic acid transfection.
[1] For the purposes of genome editing, Cas9 protein combined with sgRNA has been delivered by methods ranging from electroporation, microinjection, lipid nanoparticle formulations, osmotically induced endocytosis followed by endosome disruption, microfluidic deformation, and cell penetrating peptides among others.
[1] Small molecules requiring intracellular delivery include: An example of the former is bleomycin, an anticancer drug with poor permeability due to its positive charge and hydrophobicity.
[36] For example, using microinjected PEGylated tracer beads of up to 5.6 micron, it was shown that motor-driven cytoplasmic mixing substantially enhanced intracellular movement of both small and large cellular components.
[45] HSC-based gene therapies prepared with gamma retroviral and lentiviral vectors have in some cases shown an increased risk of leukemia down the track due to genotoxicity,[46] as occurred with Strimvelis.
[50] There is currently little data available on the medical impact of intracellular delivery of novel chemical components of mRNA vaccines, such as SM-102 and ALC-0315, on both the short and long-term health of the recipient population.
The major challenge for membrane disruption-mediated methods is to create holes of the optimal shape, size, location, and duration for the required delivery application.
[53] The internalization pathways employed by target cells depend on the size, shape, material composition, surface chemistry, and/or charge of the carrier .
In these cases, juxtaposed membranes are pulled into close contact by specific protein–protein interactions and interfacial water is excluded to promote lipid mixing and subsequent fusion.
Enveloped viruses may employ transmembrane viral proteins to mediate fusion with target cell membranes and this mechanism has been exploited for engineered intracellular delivery .
[77] The nano and micro versions of electroporation feature much higher precision and control over the size and location of membrane disruptions imposed on target cells .
[78] A company called Maxcyte has developed a high-throughput version of flow electroporation that can process hundreds of millions of cells in tens of minutes .
[87][88] The method, termed cell squeezing, was spun out into a company called SQZ biotech that focuses on leveraging intracellular delivery technology to develop cell-based therapies.
Since the 1990s, more precise strategies to employ fluid shear forces to permeabilize cells include microfluidics, ultrasound, shock waves, and laser-based methods .
[1] Laser irradiance of an absorbent object in an aqueous environment can produce a variety of effects including cavitation, plasma production, chemical reactions, and heat .
For example, a metallic nanostructure can be used as a seed structure to harvest short laser pulse energy and convert it into highly localized explosive vapor bubbles.
A high throughput version of this concept was unveiled in 2015 [95] Substrates arrayed with pores lined by metallic absorbers were irradiated to generate exploding cavitation bubbles underneath the basal side of adherent cells.
Membrane permeabilization was synchronized with active pumping of cargo through the pores to successfully introduce living bacteria (>1 micron) into the cytoplasm of several cell types.
Gold nanoparticles were packed into a dense surface layer where >10 s of infrared laser irradiation heats the underside of cells to trigger permeabilization and delivery of dyes, dextrans and plasmids.
[100] Diverse ligands including small molecules, carbohydrates, aptamers, peptides and antibodies have been covalently linked to siRNA in order to improve cellular uptake and target specific cell types.
[101] GalNAc-siRNA conjugates were employed in the second FDA-approved siRNA medicine, Givosiran, which is administered to treat acute Hepatic Porphyria by down-regulating ALAS1 expression in the liver.
[43] Chemical modifications of ASO nucleosides, nucleobases, and the internucleoside backbone are key for improving pharmacokinetics and pharmacodynamics while maintaining target affinity and efficacy.
Most medically applicable ASOs are naked molecules that are able to enter cells through endocytosis and exert their therapeutic effects by binding their intracellular target .
Because VLPs are derived from existing viral scaffolds, they exploit natural properties of viruses that enable efficient intracellular delivery, including their ability to encapsulate cargos, escape endosomes, and be reprogrammed to target different cell types.
However, unlike viruses, VLPs can deliver their cargo as mRNA or protein instead of as DNA, which substantially reduces the risks of viral genome integration.