Iodine heptafluoride
Iodine heptafluoride is an interhalogen compound with the chemical formula IF7.
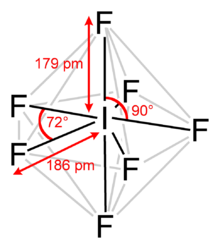
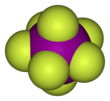
[2][3] It has an unusual pentagonal bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory.
However, this melting is difficult to observe, as the liquid form is thermodynamically unstable at 760 mmHg: instead, the compound begins to sublime at 4.77 °C.
Alternatively, this compound can be prepared from fluorine and dried palladium or potassium iodide to minimize the formation of IOF5, an impurity arising by hydrolysis.
It also is a strong oxidizer and can cause fire on contact with organic material.