Ionic crystal
They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice.
The properties of NaCl reflect the strong interactions that exist between the ions.
It is a good conductor of electricity when molten, but very poor in the solid state.
When fused the mobile ions carry charge through the liquid.
[2] They are characterized by strong absorption of infrared radiation and have planes along which they cleave easily.
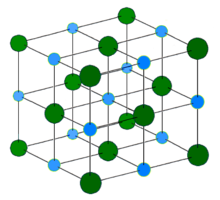
Na
+
Cl
−