Potassium iodide
[4][5] It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are used.
[6] Common side effects include vomiting, diarrhea, abdominal pain, rash, and swelling of the salivary glands.
In humans it is the most common additive used for iodizing table salt (a public health measure to prevent iodine deficiency in populations that get little seafood).
The oxidation of iodide causes slow loss of iodine content from iodised salts that are exposed to excess air.
Dextrose or sodium thiosulfate are often added to iodized table salt to stabilize potassium iodide thus reducing loss of the volatile chemical.
[15] Thyroid iodine uptake blockade with potassium iodide is used in nuclear medicine scintigraphy and therapy with some radioiodinated compounds that are not targeted to the thyroid, such as iobenguane (MIBG), which is used to image or treat neural tissue tumors, or iodinated fibrinogen, which is used in fibrinogen scans to investigate clotting.
[16] However, in cases of prolonged or repeated exposure, health authorities may recommend multiple daily doses.
[16] Priority for prophylaxis is given to the most sensitive groups: pregnant and breastfeeding women, infants, and children under 18 years.
[23] In 1982, the U.S. Food and Drug Administration approved potassium iodide to protect thyroid glands from radioactive iodine involving accidents or fission emergencies.
[25] By saturating the body with a source of stable iodide prior to exposure, inhaled or ingested 131I tends to be excreted, which prevents radioiodine uptake by the thyroid.
In contrast, KI administration after exposure to radioiodine induces a smaller and rapidly decreasing blockade effect.
Since KI protects for approximately 24 hours, it must be dosed daily until a risk of significant exposure to radioiodine no longer exists.
Potassium iodide is also not recommended for people with dermatitis herpetiformis and hypocomplementemic vasculitis – conditions that are linked to a risk of iodine sensitivity.
The anti-radioiodine doses used for 131I uptake blockade are lower, and range downward from 100 mg a day for an adult, to less than this for children (see table).
More severe side effects that require notification of a physician are: fever, weakness, unusual tiredness, swelling in the neck or throat,[citation needed] mouth sores, skin rash, nausea, vomiting, stomach pains, irregular heartbeat,[citation needed] numbness or tingling of the hands or feet, or a metallic taste in the mouth.
However, in the event of a radioiodine release too massive and widespread to be controlled by the limited stock of iodide and iodate prophylaxis drugs, then the addition of perchlorate ions to the water supply, or distribution of perchlorate tablets would serve as a cheap, efficacious, second line of defense against carcinogenic radioiodine bioaccumulation.
[35] Potassium iodide is a component in the electrolyte of dye sensitised solar cells (DSSC) along with iodine.
Aged and impure samples are yellow because of the slow oxidation of the salt to potassium carbonate and elemental iodine.
Air will oxidize iodide, as evidenced by the observation of a purple extract when aged samples of KI are rinsed with dichloromethane.
Potassium iodide's (KI) value as a radiation protective (thyroid blocking) agent was demonstrated following the Chernobyl nuclear reactor disaster in April 1986.
Within ten years of the accident, it became clear that thyroid damage caused by released radioactive iodine was virtually the only adverse health effect that could be measured.
Researchers at the World Health Organization accurately located and counted the residents with cancer from Chernobyl and were startled to find that "the increase in incidence [of thyroid cancer] has been documented up to 500 km from the accident site... significant doses from radioactive iodine can occur hundreds of kilometers from the site, beyond emergency planning zones.
[47] An editorial in The Journal of the American Medical Association regarding thyroid diseases in both hibakusha and those affected by the Chernobyl disaster reports that "[a] straight line adequately describes the relationship between radiation dose and thyroid cancer incidence" and states "it is remarkable that a biological effect from a single brief environmental exposure nearly 60 years in the past is still present and can be detected.
Naval air crew members flying within 70 nautical miles of the Fukushima Daiichi Nuclear Power Plant damaged in the earthquake (8.9/9.0 magnitude) and ensuing tsunami on 11 March 2011.
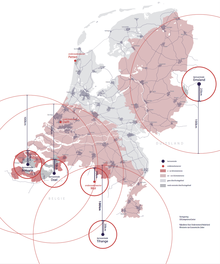
In 2017, the Dutch government distributed pills to hundreds of thousands of residents who lived within a certain distance of nuclear power plants and met some other criteria.
[51][52] By 2020, potassium iodide tablets are made available free of charge for all residents in all pharmacies throughout the country.
[53] Three companies (Anbex, Inc., Fleming Co, and Recipharm of Sweden) have met the strict FDA requirements for manufacturing and testing of KI, and they offer products (IOSAT, ThyroShield, and ThyroSafe,[54] respectively) which are available for purchase.
Tablets of potassium iodide are supplied for emergency purposes related to blockade of radioiodine uptake, a common form of radiation poisoning due to environmental contamination by the short-lived fission product 131I.
For reasons noted above, therapeutic drops of SSKI, or 130 mg tablets of KI as used for nuclear fission accidents, are not used as nutritional supplements, since an SSKI drop or nuclear-emergency tablet provides 300 to 700 times more iodine than the daily adult nutritional requirement.