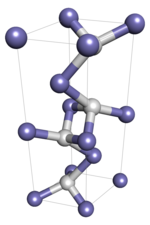
Iron(I) hydride
However, as the name is compositional in nature, it does not distinguish between compounds of the same stoichiometry, such as molecular species, which exhibit distinct chemical properties.
It has been detected in isolation only in extreme environments, like trapped in frozen noble gases, in the atmosphere of cool stars, or as a gas at temperatures above the boiling point of iron.
It is assumed to have three dangling valence bonds, and is therefore a free radical; its formula may be written FeH3• to emphasize this fact.
Since it is a polymeric solid, a monocrystalline sample is not expected to undergo state transitions, such as melting and dissolution, as this would require the rearrangement of molecular bonds and consequently, change its chemical identity.
Colloidal crystalline samples, wherein intermolecular forces are relevant, are expected to undergo state transitions.
It is predicted to exhibit polymorphism, transitioning at some temperature below −173 °C (−279 °F) to a face-centred crystalline structure with the Fm3m space group.
[6] The small isotope shift of the deuterated FeD compared to FeH at this wavelength shows that the band is due to a (0,0) transition from the ground state, namely F4Δ—X4Δ.
[7] Elliptical and lenticular galaxies also have an observable Wing-Ford band, due to a large amount of their light coming from M dwarfs.
[14] Kleman and Åkerlind first produced FeH in the laboratory by heating iron to 2600 K in a King-type furnace under a thin hydrogen atmosphere.
Molecular FeH can also be obtained (together with FeH2 and other species) by vaporizing iron in an argon-hydrogen atmosphere and freezing the gas on a solid surface at about 10 K (-263 °C).
The compound can be detected by infrared spectroscopy, and about half of it disappears when the sample is briefly warmed to 30 K.[15] A variant technique uses pure hydrogen atmosphere condensed at 4 K.[1] This procedure also generates molecules that were thought to be FeH3 (ferric hydride) but were later assigned to an association of FeH and molecular hydrogen H2.
Mössbauer spectroscopy revealed an isomer shift of 0.59 mm/s compared with metallic iron and quadrupole splitting of 2.4 mm/s.
[17] FeH can also be produced by the interaction of Iron pentacarbonyl vapour and atomic hydrogen in a microwave discharge.