Isophorone
It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish.
[4] Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone.
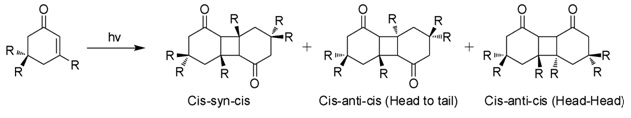
[6] When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure).
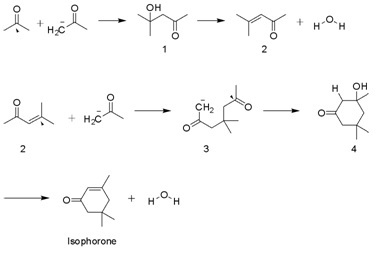
[8] Isophorone is produced on a multi-thousand ton scale by the aldol condensation of acetone using KOH.
[11] The use of isophorone as a solvent resulted from the search for ways to dispose of or recycle acetone, which is a waste product of phenol synthesis by the Hock method.