Aldol condensation
Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds.
The process begins when a free hydroxide (strong base) strips the highly acidic proton at the alpha carbon of the aldehyde.
In the second part of the reaction, the presence of base leads to elimination of water and formation of a new C–C pi bond.
Ordinarily, this leads to four possible products as either carbonyl compound can act as the nucleophile and self-condensation is possible, which makes a synthetically useless mixture.
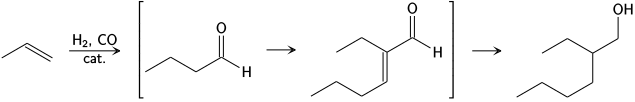
[12] The Aldox process, developed by Royal Dutch Shell and Exxon, converts propene and syngas to 2-ethylhexanol via hydroformylation to butyraldehyde, aldol condensation to 2-ethylhexanal and finally hydrogenation.
[13] Pentaerythritol is produced on a large scale beginning with crossed aldol condensation of acetaldehyde and three equivalents of formaldehyde to give pentaerythrose, which is further reduced in a Cannizzaro reaction.
Ethyl glyoxylate 2 and glutaconate (diethyl-2-methylpent-2-enedioate) 1 react to isoprenetricarboxylic acid 3 (isoprene (2-methylbuta-1,3-diene) skeleton) with sodium ethoxide.
This obstacle is overcome by using a strong base such as potassium hydroxide and a very polar solvent such as DMSO in the reaction below:[19] The product can epimerize by way of a common intermediate—enolate A—to convert between the original (S,R) and the (R,R) epimers.