J chain
An N-linked carbohydrate resulting from N-glycosylation is also essential in the protein's incorporation to antibody polymers.
[15] However, J chain-KO mice have been shown have low concentrations of hexameric IgM and a deficiency in complement activation, suggesting additional in vivo regulatory mechanisms.
Structurally, the J chain joins two antibody monomers asymmetrically by forming intermolecular disulfide bonds and bringing hydrophobic β-sandwiches on each molecule together.
A basal protein of the pIgR known as secretory component (SC) recognizes Ig ready for secretion.
[21] The complex is then transcytosed and the secretory component proteolytically cleaved from the receptor releasing the antibody to the apical side of the epithelial cell and to the lumen at large.
[24] As Pax5 is a common transcriptional regulator, the J chain is still expressed in plasma cells that secrete monomeric antibodies.
[28] This makes sharks an intriguing model organism in studying J chain regulation and polymerization without the confounding variables of mucosal secretion.
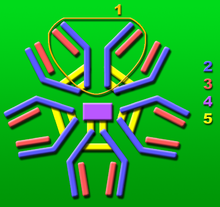
1: Base unit.
2: Heavy chains .
3: Light chains .
4: J chain .
5: Intermolecular disulfide bonds.
