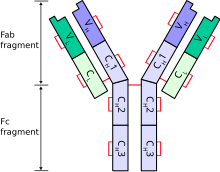
Immunoglobulin heavy chain
[3] There are five types of mammalian immunoglobulin heavy chain: γ, δ, α, μ and ε.
Each heavy chain has two regions: Cows, specifically Bos taurus, show a variation on the general mammalian theme in which the heavy chain CDR H3 region has adapted to produce a divergent repertoire of antibodies which present a "stalk and knob" antigen interaction surface instead of the more familiar bivalent tip surface.
[7] The bovine CDR is unusually long and contains unique sequence attributes which support the production of paired cysteine residues during somatic hypermutation.
[7] A speculated evolutionary driver for this variation is the presence of a vastly more diverse microbial environment in the digestive system of the cow as a consequence of their being ruminants.
The resulting antibodies are designated IgW (also called IgX or IgNARC) and IgNAR (immunoglobulin new antigen receptor).