Kiliani–Fischer synthesis
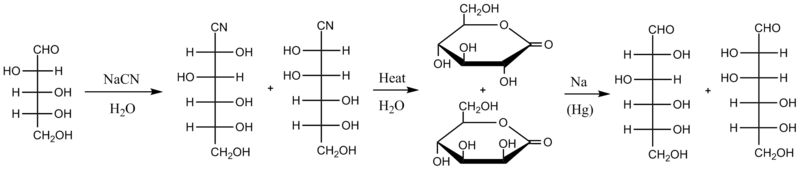
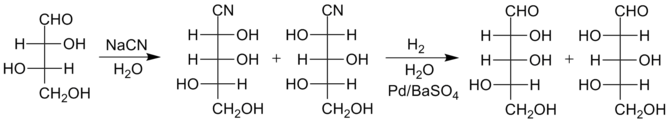
The Kiliani–Fischer synthesis, named for German chemists Heinrich Kiliani and Emil Fischer, is a method for synthesizing monosaccharides.
The new chiral carbon is produced with both stereochemistries, so the product of a Kiliani–Fischer synthesis is a mixture of two diastereomeric sugars, called epimers.
The cyanohydrin resulting from this addition is heated in water, which hydrolyzes the cyanide into a carboxylic acid group that quickly reacts with itself to form a more stable lactone.
Due to the presence of water, the imine quickly hydrolyzes to form an aldehyde, thus the final sugars are produced in just two steps rather than three.
The next iteration leads to the hexoses D-allose (4a) and D-altrose (4b), D-glucose (4c) and D-mannose (4d), D-gulose (4e) and D-idose (4f), and D-galactose (4g) and D-talose (4h).
While it does provide access to every possible stereoisomer of any desired aldose, the process is limited by its low yield and use of toxic reagents.