Enol
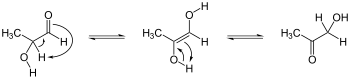
[1] Keto–enol tautomerism refers to a chemical equilibrium between a "keto" form (a carbonyl, named for the common ketone case) and an enol.
The interconversion of the two forms involves the transfer of an alpha hydrogen atom and the reorganisation of bonding electrons.
[2] Organic esters, ketones, and aldehydes with an α-hydrogen (C−H bond adjacent to the carbonyl group) often form enols.
The acid-catalyzed conversion of an enol to the keto form proceeds by proton transfer from O to carbon.
Depending on the nature of the three R groups, the resulting products in this situation would be diastereomers or enantiomers.
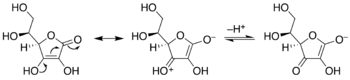
[citation needed] Enediols are alkenes with a hydroxyl group on each carbon of the C=C double bond.
In the Calvin cycle, the ribulose equilibrates with the enediol, which then binds carbon dioxide.
The same enediol is also susceptible to attack by oxygen (O2) in the (undesirable) process called photorespiration.
[citation needed] The enzyme enolase catalyzes the dehydration of 2-phosphoglyceric acid to the enol phosphate ester.