Crystallographic defect
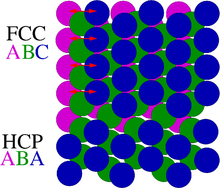
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids.
These dislocations permit ionic transport through crystals leading to electrochemical reactions.
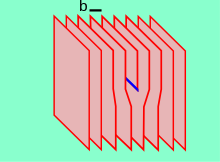
Edge dislocations are caused by the termination of a plane of atoms in the middle of a crystal.
In metallic materials, b is aligned with close-packed crystallographic directions and its magnitude is equivalent to one interatomic spacing.
It is the presence of dislocations and their ability to readily move (and interact) under the influence of stresses induced by external loads that leads to the characteristic malleability of metallic materials.
Deep-level transient spectroscopy has been used for studying the electrical activity of dislocations in semiconductors, mainly silicon.
[15] A successful mathematical classification method for physical lattice defects, which works not only with the theory of dislocations and other defects in crystals but also, e.g., for disclinations in liquid crystals and for excitations in superfluid 3He, is the topological homotopy theory.