Lead(II) fluoride
Lead(II) fluoride is the inorganic compound with the formula PbF2.
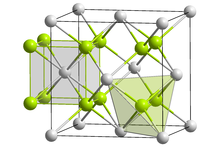
The compound is polymorphic, at ambient temperatures it exists in orthorhombic (PbCl2 type) form, while at high temperatures it is cubic (Fluorite type).
[5][6] Lead(II) fluoride is used in low melting glasses, in glass coatings to reflect infrared rays, in phosphors for television-tube screens, and as a catalyst for the manufacture of picoline.
[3] The Muon g−2 experiment uses PbF2 scintillators in conjunction with silicon photomultipliers.
[7] It also serves as a oxygen scavenger in high-temperature fluorine chemistry, as plumbous oxide is relatively volatile.

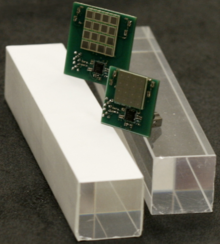
2 scintillator crystals used in the Muon g−2 experiment.