Leishmaniasis
Leishmaniasis is a wide array of clinical manifestations caused by protozoal parasites of the Trypanosomatida genus Leishmania.
[7] It is generally spread through the bite of phlebotomine sandflies, Phlebotomus and Lutzomyia, and occurs most frequently in the tropics and sub-tropics of Africa, Asia, the Americas, and southern Europe.
The visceral form starts with skin ulcers and later presents with fever, low red blood cell count, and enlarged spleen and liver.
[2] Other measures include spraying insecticides to kill sandflies and treating people with the disease early to prevent further spread.
[2][12] About 200 million people in Asia, Africa, South and Central America, and southern Europe live in areas where the disease is common.
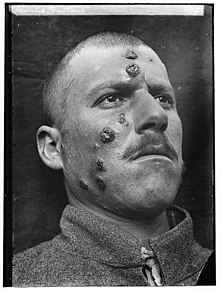
[2] The symptoms of leishmaniasis are skin sores which erupt weeks to months after the person is bitten by infected sandflies.
Leishmaniasis is considered one of the classic causes of a markedly enlarged (and therefore palpable) spleen; the organ, which is not normally felt during the examination of the abdomen, may even become larger than the liver in severe cases.
The genomes of three Leishmania species (L. major, L. infantum, and L. braziliensis) have been sequenced, and this has provided much information about the biology of the parasite.
For example, in Leishmania, protein-coding genes are understood to be organized as large polycistronic units in a head-to-head or tail-to-tail manner; RNA polymerase II transcribes long polycistronic messages in the absence of defined RNA pol II promoters, and Leishmania has unique features concerning the regulation of gene expression in response to changes in the environment.
In one study, L. major was identified in twelve out of ninety-one wild western lowland gorilla fecal samples[17] and in a study of fifty-two captive non-human primates under zoo captivity in a leishmaniasis endemic area, eight (all three chimpanzees, three golden lion tamarins, a tufted capuchin, and an Angolan talapoin), were found to be infected with L. infantum and capable of infecting Lutzomyia longipalpis sand flies, although "parasite loads in infected sand flies observed in this study were considered low".
So, other indirect immunological methods of diagnosis are developed, including enzyme-linked immunosorbent assay, antigen-coated dipsticks, and direct agglutination test.
[2] For visceral leishmaniasis in India, South America, and the Mediterranean, liposomal amphotericin B is the recommended treatment and is often used as a single dose.
[25] No studies have determined the effect of oral nutritional supplements on visceral leishmaniasis being treated with anti-leishmanial drug therapy.
[28] The settings in which leishmaniasis is found range from rainforests in Central and South America to deserts in western Asia and the Middle East.
[34] Leishmaniasis is also known as papalomoyo, papa lo moyo, úlcera de los chicleros, and chiclera in Latin America.
[35] During 2004, an estimated 3,400 troops from the Colombian army, operating in the jungles near the south of the country (in particular around the Meta and Guaviare departments), were infected with leishmaniasis.
Allegedly, a contributing factor was that many of the affected soldiers did not use the officially provided insect repellent because of its disturbing odor.
Within Afghanistan, leishmaniasis occurs commonly in Kabul, partly due to bad sanitation and waste left uncollected in streets, allowing parasite-spreading sand flies an environment they find favorable.
Persian physicians, including Avicenna in the 10th century CE, gave detailed descriptions of what was called balkh sore.
In the Americas, evidence of the cutaneous form of the disease in Ecuador and Peru appears in pre-Inca pottery depicting skin lesions and deformed faces dating back to the first century CE.
[51][52] Peter Borovsky, a Russian military surgeon working in Tashkent, conducted research into the etiology of "oriental sore", locally known as sart sore, and in 1898 published the first accurate description of the causative agent, correctly described the parasite's relation to host tissues and correctly referred it to the protozoa.
[54] A few months later, Captain Charles Donovan (1863–1951) confirmed the finding of what became known as Leishman-Donovan bodies in smears taken from people in Madras in southern India.
[55] But it was Ronald Ross who proposed that Leishman-Donovan bodies were the intracellular stages of a new parasite, which he named Leishmania donovani.
[57] Transmission by the sandfly was hypothesized by Lionel Napier and Ernest Struthers at the School of Tropical Medicine at Calcutta and later proven by his colleagues.
[58][59] The disease became a major problem for Allied troops fighting in Sicily during the Second World War; research by Leonard Goodwin then showed pentostam was an effective treatment.