Retinal
One study suggests that approximately three billion years ago, most living organisms on Earth used retinal, rather than chlorophyll, to convert sunlight into energy.
Retinal was originally called retinene,[3] and was renamed[4] after it was discovered to be vitamin A aldehyde.
Invertebrates such as insects and squid use hydroxylated forms of retinal in their visual systems, which derive from conversion from other xanthophylls.
This configuration change pushes against an opsin protein in the retina, which triggers a chemical signaling cascade, which results in perception of light or images by the brain.
The cones form incomplete disks that are part of the plasma membrane, so that the N-terminus head extends outside of the cell.
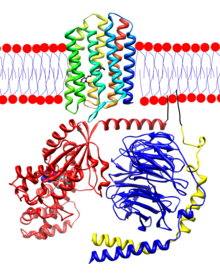
In opsins, retinal binds covalently to a lysine[16] in the seventh transmembrane helix[17][18][19] through a Schiff base.
[22] Cattle rhodopsin, the opsin of the rod cells, was the first GPCR to have its amino acid sequence[23] and 3D-structure (via X-ray crystallography) determined.
With the exception of the dipteran suborder Cyclorrhapha (the so-called higher flies), all insects examined use the (R)-enantiomer of 3-hydroxyretinal.
[32] All-trans-retinal is also an essential component of microbial opsins such as bacteriorhodopsin, channelrhodopsin, and halorhodopsin, which are important in bacterial and archaeal anoxygenic photosynthesis.
These proteins are not evolutionarily related to animal opsins and are not GPCRs; the fact that they both use retinal is a result of convergent evolution.
For his work, Wald won a share of the 1967 Nobel Prize in Physiology or Medicine with Haldan Keffer Hartline and Ragnar Granit.