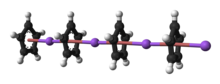
Lithium cyclopentadienide
Lithium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
It is prepared by treating cyclopentadiene with butyllithium:[1] Because lithium cyclopentadienide is usually handled as a solution, the solvent-free solid is rarely encountered.
According to X-ray crystallography, LiCp is a "polydecker" sandwich complex, consisting of an infinite chain of alternating Li+ centers sandwiched between μ-η5:η5-C5H5 ligands.
[1] LiCp is a common reagent for the preparation of cyclopentadienyl complexes.
MgCpBr (TiCp2Cl)2 TiCpCl3 TiCp2S5 TiCp2(CO)2 TiCp2Me2 VCpCh VCp2Cl2 VCp(CO)4 (CrCp(CO)3)2 Fe(η5-C5H4Li)2 ((C5H5)Fe(C5H4))2 (C5H4-C5H4)2Fe2 FeCp2PF6 FeCp(CO)2I CoCp(CO)2 NiCpNO ZrCp2ClH MoCp2Cl2 (MoCp(CO)3)2 RuCp(PPh3)2Cl RuCp(MeCN)3PF6