Ernest Rutherford
[11] He arrived at this theory through his discovery and interpretation of Rutherford scattering during the gold foil experiment performed by Hans Geiger and Ernest Marsden.
In 1917, he performed the first artificially induced nuclear reaction by conducting experiments in which nitrogen nuclei were bombarded with alpha particles.
These experiments led him to discover the emission of a subatomic particle that he initially called the "hydrogen atom", but later (more precisely) renamed the proton.
In the same year, the first controlled experiment to split the nucleus was performed by John Cockcroft and Ernest Walton, working under his direction.
[14] He was the fourth of twelve children of James Rutherford, an immigrant farmer and mechanic from Perth, Scotland, and his wife Martha Thompson, a schoolteacher from Hornchurch, England.
[16] At Canterbury, he was awarded a complex BA in Latin, English, and Maths in 1892, a MA in Mathematics and Physical Science in 1893, and a BSc in Chemistry and Geology in 1894.
[22][23] Thereafter, he invented a new form of radio receiver, and in 1895 Rutherford was awarded an 1851 Research Fellowship from the Royal Commission for the Exhibition of 1851,[24][25] to travel to England for postgraduate study at the Cavendish Laboratory, University of Cambridge.
[28][29] Hearing of Henri Becquerel's experience with uranium, Rutherford started to explore its radioactivity, discovering two types that differed from X-rays in their penetrating power.
[30] In 1898, Rutherford was accepted to the chair of Macdonald Professor of physics position at McGill University in Montreal, Canada, on Thomson's recommendation.
[22] In 1903, Rutherford considered a type of radiation, discovered (but not named) by French chemist Paul Villard in 1900, as an emission from radium, and realised that this observation must represent something different from his own alpha and beta rays, due to its very much greater penetrating power.
In 1904, Rutherford suggested that radioactivity provides a source of energy sufficient to explain the existence of the Sun for the many millions of years required for the slow biological evolution on Earth proposed by biologists such as Charles Darwin.
[33] Later that year, he was elected as a member to the American Philosophical Society,[34] and in 1907 he returned to Britain to take the chair of physics at the Victoria University of Manchester.
In conjunction with Hans Geiger, he developed zinc sulfide scintillation screens and ionisation chambers to count alpha particles.
[36][37]: 61 In late 1907, Ernest Rutherford and Thomas Royds allowed alphas to penetrate a very thin window into an evacuated tube.
[37]: 63 Under his direction in 1909, Hans Geiger and Ernest Marsden performed the Geiger–Marsden experiment, which demonstrated the nuclear nature of atoms by measuring the deflection of alpha particles passing through a thin gold foil.
[42] Rutherford was inspired to ask Geiger and Marsden in this experiment to look for alpha particles with very high deflection angles, which was not expected according to any theory of matter at that time.
[49][50] It was not until 1919 that Rutherford expanded upon his theory of the "positive electron" with a series of experiments beginning shortly before the end of his time at Manchester.
He found that nitrogen, and other light elements, ejected a proton, which he called a "hydrogen atom", when hit with α (alpha) particles.
[52] During his tenure, Nobel prizes were awarded to James Chadwick for discovering the neutron (in 1932), John Cockcroft and Ernest Walton for an experiment that was to be known as splitting the atom using a particle accelerator, and Edward Appleton for demonstrating the existence of the ionosphere.
In 1919–1920, Rutherford continued his research on the "hydrogen atom" to confirm that alpha particles break down nitrogen nuclei and to affirm the nature of the products.
[55] In Rutherford's four-part article on the "Collision of α-particles with light atoms" he reported two additional fundamental and far reaching discoveries.
[59] In 1933, Rutherford was one of the two inaugural recipients of the T. K. Sidey Medal, which was established by the Royal Society of New Zealand as an award for outstanding scientific research.
[22] For some time before his death, Rutherford had a small hernia, which he neglected to have repaired, and it eventually became strangulated, rendering him violently ill.
He had an emergency operation in London, but died in Cambridge four days later, on 19 October 1937, at age 66, of what physicians termed "intestinal paralysis".
[71] After cremation at Golders Green Crematorium,[71] he was given the high honour of burial in Westminster Abbey, near Isaac Newton, Charles Darwin, and other illustrious British scientists.
[22][72] At the opening session of the 1938 Indian Science Congress, which Rutherford had been expected to preside over before his death, astrophysicist James Jeans spoke in his place and deemed him "one of the greatest scientists of all time", saying: In his flair for the right line of approach to a problem, as well as in the simple directness of his methods of attack, [Rutherford] often reminds us of Faraday, but he had two great advantages which Faraday did not possess, first, exuberant bodily health and energy, and second, the opportunity and capacity to direct a band of enthusiastic co-workers.
[7][74][28] Patrick Blackett, a research fellow working under Rutherford, using natural alpha particles, demonstrated induced nuclear transmutation.
However, a speech of Rutherford's about his artificially-induced transmutation in lithium, printed in the 12 September 1933 issue of The Times, was reported by Szilárd to have been his inspiration for thinking of the possibility of a controlled energy-producing nuclear chain reaction.
[79] Andrew Hodwitz portrays Rutherford in episode 11 of season 13 "Staring Blindly into the Future" (January 13, 2020) of the Canadian television period detective series Murdoch Mysteries.

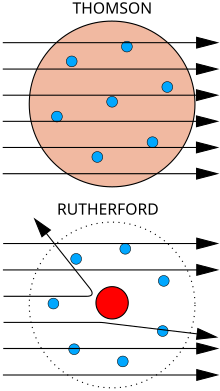
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated charge . Diagram is not to scale; in reality the nucleus is vastly smaller than the electron shell.
