Lyman continuum photons
Working from Victor Schumann's discovery of ultraviolet light, from 1906 to 1914, Theodore Lyman observed that atomic hydrogen absorbs light only at specific frequencies (or wavelengths) and the Lyman series is thus named after him.
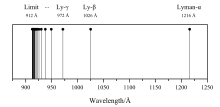
In the case of neutral atomic hydrogen, the minimum ionization energy is equal to the Lyman limit, where the photon has enough energy to completely ionize the atom, resulting in a free proton and a free electron.
[3][4] The Lyman limit is at the wavelength of 91.2 nm (912 Å), corresponding to a frequency of 3.29 million GHz and a photon energy of 13.6 eV.
[3] LyC energies are mostly in the ultraviolet C portion of the electromagnetic spectrum (see Lyman series).
Although X-rays and gamma-rays will also ionize a hydrogen atom, there are far fewer of them emitted from a star's photosphere—LyC are predominantly UV-C.