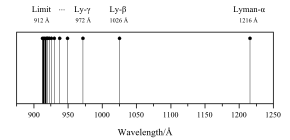
Lyman series
In physics and chemistry, the Lyman series is a hydrogen spectral series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1 (where n is the principal quantum number), the lowest energy level of the electron (groundstate).
The transitions are named sequentially by Greek letters: from n = 2 to n = 1 is called Lyman-alpha, 3 to 1 is Lyman-beta, 4 to 1 is Lyman-gamma, and so on.
The greater the difference in the principal quantum numbers, the higher the energy of the electromagnetic emission.
The first line in the spectrum of the Lyman series was discovered in 1906 by physicist Theodore Lyman IV, who was studying the ultraviolet spectrum of electrically excited hydrogen gas.
The rest of the lines of the spectrum (all in the ultraviolet) were discovered by Lyman from 1906-1914.
Within five years Johannes Rydberg came up with an empirical formula that solved the problem, presented first in 1888 and final form in 1890.
Rydberg managed to find a formula to match the known Balmer series emission lines, and also predicted those not yet discovered.
On December 1, 2011, it was announced that Voyager 1 detected the first Lyman-alpha radiation originating from the Milky Way galaxy.
The wavelengths in the Lyman series are all ultraviolet: In 1914, when Niels Bohr produced his Bohr model theory, the reason why hydrogen spectral lines fit Rydberg's formula was explained.
This also means that the inverse of the Rydberg constant is equal to the Lyman limit.