Electronvolt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an electric potential difference of one volt in vacuum.
When used as a unit of energy, the numerical value of 1 eV in joules (symbol J) is equal to the numerical value of the charge of an electron in coulombs (symbol C).
Under the 2019 revision of the SI, this sets 1 eV equal to the exact value 1.602176634×10−19 J.
[1] Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge q gains an energy E = qV after passing through a voltage of V. An electronvolt is the amount of energy gained or lost by a single electron when it moves through an electric potential difference of one volt.
Hence, it has a value of one volt, which is 1 J/C, multiplied by the elementary charge e = 1.602176634×10−19 C.[2] Therefore, one electronvolt is equal to 1.602176634×10−19 J.
It is a commonly used unit of energy within physics, widely used in solid state, atomic, nuclear and particle physics, and high-energy astrophysics.
It is commonly used with SI prefixes milli- (10−3), kilo- (103), mega- (106), giga- (109), tera- (1012), peta- (1015) or exa- (1018), the respective symbols being meV, keV, MeV, GeV, TeV, PeV and EeV.
In some older documents, and in the name Bevatron, the symbol BeV is used, where the B stands for billion.
The symbol BeV is therefore equivalent to GeV, though neither is an SI unit.
By mass–energy equivalence, the electronvolt corresponds to a unit of mass.
It is common in particle physics, where units of mass and energy are often interchanged, to express mass in units of eV/c2, where c is the speed of light in vacuum (from E = mc2).
It is common to informally express mass in terms of eV as a unit of mass, effectively using a system of natural units with c set to 1.
For example, an electron and a positron, each with a mass of 0.511 MeV/c2, can annihilate to yield 1.022 MeV of energy.
To convert to electronvolt mass-equivalent, use the formula: By dividing a particle's kinetic energy in electronvolts by the fundamental constant c (the speed of light), one can describe the particle's momentum in units of eV/c.
[5] In natural units in which the fundamental velocity constant c is numerically 1, the c may informally be omitted to express momentum using the unit electronvolt.
in high-energy physics such that an applied energy with expressed in the unit eV conveniently results in a numerically approximately equivalent change of momentum when expressed with the unit eV/c.
Dividing a unit of energy (such as eV) by a fundamental constant (such as the speed of light) that has the dimension of velocity (T−1L) facilitates the required conversion for using a unit of energy to quantify momentum.
For example, if the momentum p of an electron is 1 GeV/c, then the conversion to MKS system of units can be achieved by:
In particle physics, a system of natural units in which the speed of light in vacuum c and the reduced Planck constant ħ are dimensionless and equal to unity is widely used: c = ħ = 1.
Outside this system of units, the conversion factors between electronvolt, second, and nanometer are the following:
The above relations also allow expressing the mean lifetime τ of an unstable particle (in seconds) in terms of its decay width Γ (in eV) via Γ = ħ/τ.
In certain fields, such as plasma physics, it is convenient to use the electronvolt to express temperature.
The electronvolt is divided by the Boltzmann constant to convert to the Kelvin scale:
The kB is assumed when using the electronvolt to express temperature, for example, a typical magnetic confinement fusion plasma is 15 keV (kiloelectronvolt), which is equal to 174 MK (megakelvin).
The energy E, frequency ν, and wavelength λ of a photon are related by
A photon with a wavelength of 532 nm (green light) would have an energy of approximately 2.33 eV.
Similarly, 1 eV would correspond to an infrared photon of wavelength 1240 nm or frequency 241.8 THz.
In a low-energy nuclear scattering experiment, it is conventional to refer to the nuclear recoil energy in units of eVr, keVr, etc.
For example, the yield of a phototube is measured in phe/keVee (photoelectrons per keV electron-equivalent energy).
The relationship between eV, eVr, and eVee depends on the medium the scattering takes place in, and must be established empirically for each material.



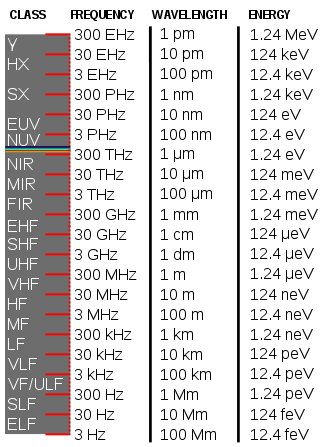
| Legend | ||
|---|---|---|
| γ: gamma rays | MIR: mid-infrared | HF: high freq. |
| HX: hard X-rays | FIR: far infrared | MF: medium freq. |
| SX: soft X-rays | radio waves | LF: low freq. |
| EUV: extreme ultraviolet | EHF: extremely high freq. | VLF: very low freq. |
| NUV: near ultraviolet | SHF: super high freq. | ULF: ultra-low freq. |
| visible light | UHF: ultra high freq. | SLF: super low freq. |
| NIR: near infrared | VHF: very high freq. | ELF: extremely low freq. |



