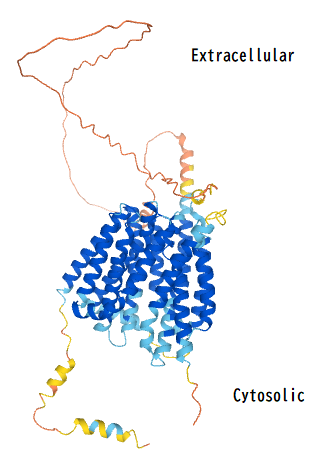
MFSD6L
The MFSD6L protein has a precursor molecular weight of approximately 64 kDa, consisting of 586 amino acids.
[5] After post-translational modifications, such as glycosylation, the mature MFSD6L protein's molecular weight increases to 72 kDa.
[13] For the part of the promoter region closest to the start of the 5' UTR of the MFSD6L gene, there were several transcription factor binding sites found.
There are conserved stem-loop regions across mammalian orthologs, which infer possible miRNA binding sites.
[16] A serine phosphorylation site at the 429th amino acid was also predicted and verified by presence within other mammalian orthologs.
[5] Through BLAST sequence analysis, the MFSD6L protein was found to have orthologs in a many mammalian species, especially among primates, and flying foxes.
[19] There were some orthologs found in the Reptilia and Amphibia classes, albeit not as great in number as in the Mammalia.
Through BLAST sequence analysis, the most distant homologs were the organisms within the Cnidaria phylum, which mainly consists of jellyfish, sea anemones, and corals.
[19] Searching with BLAST for the MFSD6L gene at an older diverging phylum, the Porifera, revealed no homologous MFSD6L protein.
This is additionally supported by the fact that the expression of MFSD6L was upregulated due to glucose starvation.A disease associated with the MFSD6L gene is the Tetralogy of Fallot, which is a series of four congenital heart defects that can cause low oxygenation of blood.
[5] This is due to a ventricular septal defect that causes the mixing of oxygenated and deoxygenated blood in the left ventricle of the heart.