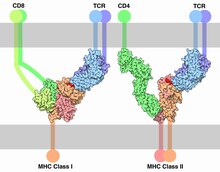
MHC restriction
The selection process results in developed T cells with specific TCRs that might only respond to certain MHC molecules but not others.
The biological reason of MHC restriction is to prevent supernumerary wandering lymphocytes generation, hence energy saving and economy of cell-building materials.
T-cells will recognize foreign peptides through T-cell receptors (TCRs) on the surface of the T cells, and then perform different roles depending on the type of T cell they are in order to defend the host from the foreign peptide, which may have come from pathogens like bacteria, viruses or parasites.
[4] Only the thymocytes (developing T cells in the thymus) that are capable of binding, with an appropriate affinity, with the MHC molecules can receive a survival signal and go on to the next level of selection.
[10] The interaction between TCRs and peptide-MHC complex is significant in maintaining the immune system against foreign antigens.
MHC restriction allows TCRs to detect host cells that are infected by pathogens, contains non-self proteins or bears foreign DNA.
Therefore, it is proposed that evolutionary pressure would lead to conserved amino acid sequences at regions of contact with MHCs on TCRs.
[12] Evidence from X-ray crystallography has shown comparable binding topologies between various TCR and MHC-peptide complexes.
[17] According to this model, T cells are capable of recognizing a variety of peptide epitopes independent of MHC molecules before undergoing thymic selection.
[19] This involves the recruitment of Lck, a tyrosine kinase essential for T cell maturation that is associated with the cytoplasmic tail of the CD4 or CD8 co-receptors.
Selection model argues that Lck is directed to TCRs by co-receptors CD4 and CD8 when they recognize MHC molecules.