Magnesite
[6] This very observation led to the postulation of a "dehydration barrier" being involved in the low-temperature formation of anhydrous magnesium carbonate.
[7] Laboratory experiments with formamide, a liquid resembling water, have shown how no such dehydration barrier can be involved.
The fundamental difficulty to nucleate anhydrous magnesium carbonate remains when using this non-aqueous solution.
Not cation dehydration, but rather the spatial configuration of carbonate anions creates the barrier in the low-temperature nucleation of magnesite.
[12] A major step forward toward the industrial production of magnesite at atmospheric pressure and a temperature of 316 K was described by Vandeginste.
Magnesite can also be formed by way of metasomatism in skarn deposits, in dolomitic limestones, associated with wollastonite, periclase, and talc.
[23][24] Similar to the production of lime, magnesite can be burned in the presence of charcoal to produce MgO, which, in the form of a mineral, is known as periclase.
Large quantities of magnesite are burnt to make magnesium oxide: an important refractory (heat-resistant) material used as a lining in blast furnaces, kilns and incinerators.
Calcination temperatures determine the reactivity of resulting oxide products and the classifications of light burnt and dead burnt refer to the surface area and resulting reactivity of the product (this is typically determined by an industry metric of the iodine number).
'Light burnt' product generally refers to calcination commencing at 450 °C and proceeding to an upper limit of 900 °C – which results in good surface area and reactivity.
Research is proceeding to evaluate the practicality of sequestering the greenhouse gas carbon dioxide in magnesite on a large scale.
[27] But the major problem is that these artificial processes require sufficient porosity-permeability so that the fluids can flow but this is hardly the case in peridotites.
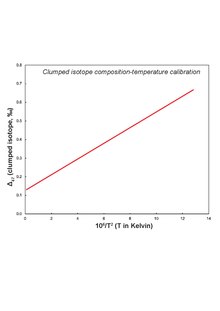
As a consequence, molecular vibration reduces and the molecule develops a lower zero point energy (see Kinetic isotope effect).
Clumped isotope thermometers have been established for carbonate minerals like dolomite,[30][31] calcite,[32] siderite[33] etc and non-carbonate compounds like methane[34] and oxygen.
The mismatch arises since bonding in magnesite is different from calcite/dolomite and/or acid digestion is conducted at higher temperature.
[41] On the other hand, coarse magnesites yield very high temperature indicating hydrothermal origin.
Magnesites forming in lakes and playa settings are in general enriched in heavy isotopes of C and O because of evaporation and CO2 degassing.
Magnesite forms as surface moulds in such conditions but more generally occur as hydrous Mg-carbonates since their precipitation is kinetically favored.
Most of the times, they derive C from DIC or nearby ultramafic complexes (e.g., Altin Playa, British Columbia, Canada[42]).
This has been verified by fluid inclusion derived temperature as well as traditional O isotope thermometry involving co-precipitating quartz-magnesite.
Recent study has shown (implementing clumped isotope thermometry) that carbonates in ALH84001 indicate formation at low temperature evaporative condition from subsurface water and derivation of CO2 from Martian atmosphere.