Nickel
Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis.
Nickel was first isolated and classified as an element in 1751 by Axel Fredrik Cronstedt, who initially mistook the ore for a copper mineral, in the cobalt mines of Los, Hälsingland, Sweden.
Major production sites include Sulawesi, Indonesia, the Sudbury region, Canada (which is thought to be of meteoric origin), New Caledonia in the Pacific, Western Australia, and Norilsk, Russia.
As a compound, nickel has a number of niche chemical manufacturing uses, such as a catalyst for hydrogenation, cathodes for rechargeable batteries, pigments and metal surface treatments.
[21] The high compressive strength of 34 GPa, predicted for ideal crystals, is never obtained in the real bulk material due to formation and movement of dislocations.
Proust analyzed samples of the meteorite from Campo del Cielo (Argentina), which had been obtained in 1783 by Miguel Rubín de Celis, discovering the presence in them of nickel (about 10%) along with iron.
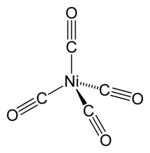
[42]Nickel tetracarbonyl (Ni(CO)4), discovered by Ludwig Mond,[43] is a volatile, highly toxic liquid at room temperature.
[45]Nickel(II) forms compounds with all common anions, including sulfide, sulfate, carbonate, hydroxide, carboxylates, and halides.
Common salts of nickel, such as chloride, nitrate, and sulfate, dissolve in water to give green solutions of the metal aquo complex [Ni(H2O)6]2+.
[58] Coins of nickel-copper alloy were minted by Bactrian kings Agathocles, Euthydemus II, and Pantaleon in the 2nd century BCE, possibly out of the Chinese cupronickel.
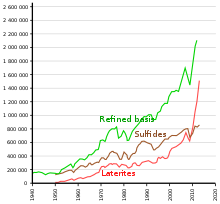
The discovery of the large deposits in the Sudbury Basin in Canada in 1883, in Norilsk-Talnakh in Russia in 1920, and in the Merensky Reef in South Africa in 1924 made large-scale nickel production possible.
Since the face value of a nickel is 5 cents, this made it an attractive target for melting by people wanting to sell the metals at a profit.
The United States Mint, anticipating this practice, implemented new interim rules on December 14, 2006, subject to public comment for 30 days, which criminalized the melting and export of cents and nickels.
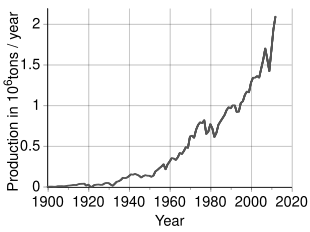
[69] An estimated 3.6 million tonnes (t) of nickel per year are mined worldwide; Indonesia (1,800,000 t), the Philippines (400,000 t), Russia (200,000 t), New Caledonia (France) (230,000 t), Canada (180,000 t) and Australia (160,000 t) are the largest producers as of 2023.
Also, extensive nickel sources are found in the depths of the Pacific Ocean, especially in an area called the Clarion Clipperton Zone in the form of polymetallic nodules peppering the seafloor at 3.5–6 km below sea level.
[79] With advances in science and engineering, regulation is currently being set in place by the International Seabed Authority to ensure that these nodules are collected in an environmentally conscientious manner while adhering to the United Nations Sustainable Development Goals.
[80] The one place in the United States where nickel has been profitably mined is Riddle, Oregon, with several square miles of nickel-bearing garnierite surface deposits.
Dicobalt octacarbonyl is also formed in nickel distillation as a by-product, but it decomposes to tetracobalt dodecacarbonyl at the reaction temperature to give a non-volatile solid.
[15] Nickel is used in many recognizable industrial and consumer products, including stainless steel, alnico magnets, coinage, rechargeable batteries (e.g. nickel–iron), electric guitar strings, microphone capsules, plating on plumbing fixtures,[94] and special alloys such as permalloy, elinvar, and invar.
Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper stainless steel (i.e., iron) alloys, except in the United States and Canada.
[66] Nickel is an excellent alloying agent for certain precious metals and is used in the fire assay as a collector of platinum group elements (PGE).
High-throughput nickel mines may also do PGE recovery (mainly platinum and palladium); examples are Norilsk, Russia and the Sudbury Basin, Canada.
Nickel makes the tungsten carbide magnetic and adds corrosion-resistance to the cemented parts, though the hardness is less than those with cobalt binder.
[102] 63Ni, with a half-life of 100.1 years, is useful in krytron devices as a beta particle (high-speed electron) emitter to make ionization by the keep-alive electrode more reliable.
[106] Nickel titanium is an alloy of roughly equal atomic percentages of its constituent metals which exhibits two closely related and unique properties: the shape memory effect and superelasticity.
[121] The US Institute of Medicine has not confirmed that nickel is an essential nutrient for humans, so neither a Recommended Dietary Allowance (RDA) nor an Adequate Intake have been established.
[122] Relatively large amounts of nickel – comparable to the estimated average ingestion above – leach into food cooked in stainless steel.
A less common form of chronic exposure is through hemodialysis as traces of nickel ions may be absorbed into the plasma from the chelating action of albumin.
Nickel is not a cumulative poison, but larger doses or chronic inhalation exposure may be toxic, even carcinogenic, and constitute an occupational hazard.
[129] Nickel compounds are classified as human carcinogens[130][131][132][133] based on increased respiratory cancer risks observed in epidemiological studies of sulfidic ore refinery workers.