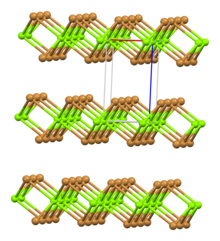
Magnesium bromide
Magnesium bromide are inorganic compounds with the chemical formula MgBr2(H2O)x, where x can range from 0 to 9.
Some magnesium bromides have been found naturally as rare minerals such as: bischofite and carnallite.
[3] It can also be made by reacting magnesium carbonate and hydrobromic acids, and collecting the solid left after evaporation.
[4] Magnesium bromide is used as a Lewis acid catalyst in some organic synthesis, e.g., in aldol reaction.
[6] Magnesium bromide modifies the catalytic properties of palladium on charcoal.