Magnesium carbonate
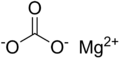
Magnesium carbonate, MgCO3 (archaic name magnesia alba), is an inorganic salt that is a colourless or white solid.
The most common magnesium carbonate forms are the anhydrous salt called magnesite (MgCO3), and the di, tri, and pentahydrates known as barringtonite (MgCO3·2H2O), nesquehonite (MgCO3·3H2O), and lansfordite (MgCO3·5H2O), respectively.
[10][11] However, calcination to the oxide is generally not considered complete below 900 °C due to interfering readsorption of liberated carbon dioxide.
[6] MgCO3 is also used in flooring, fireproofing, fire extinguishing compositions, cosmetics, dusting powder, and toothpaste.
Because of its low solubility in water and hygroscopic properties, MgCO3 was first added to table salt (NaCl) in 1911 to make it flow more freely.
[13] Powdered magnesium carbonate, known as climbing chalk or gym chalk is also used as a drying agent on athletes' hands in rock climbing, gymnastics, powerlifting, weightlifting and other sports in which a firm grip is necessary.
It can be mixed with hydrogen peroxide to create a paste, which is spread on the skull to give it a white finish.