Markó–Lam deoxygenation
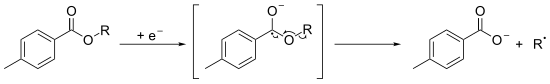
[4] The main features of the reaction are: A hydroxyl group is first derivitised into a stable and very often crystalline toluate derivative.
The aromatic ester is submitted to a monoelectronical reduction, by the use of SmI2/HMPA[5] or by electrolysis,[6] to yield the a radical-anion which decomposes into the corresponding carboxylate and into the radical of the alkyl fragment.
This radical could be used for further chemical reactions or can abstract a hydrogen atom to form the deoxygenated product.
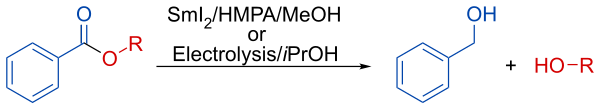
In presence of methanol or isopropanol, the reduction lead to the selective deprotection of the aromatic esters.
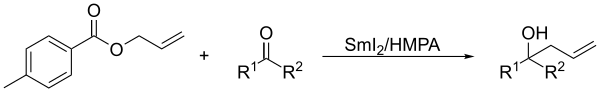
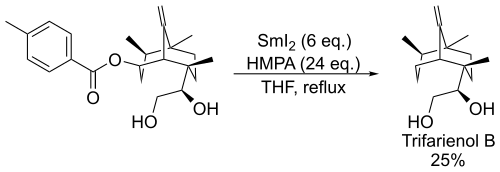
[7] In presence of ketones, allylic derivatives lead to the coupling product when treated in Barbier's conditions with samarium diiodide.