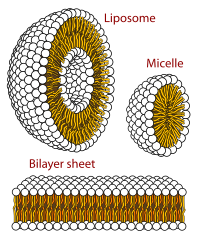
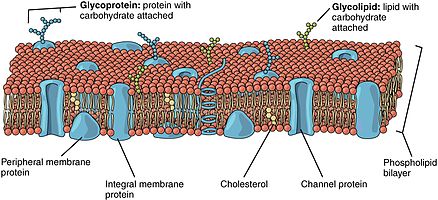
Biological membrane
The bulk of lipids in a cell membrane provides a fluid matrix for proteins to rotate and laterally diffuse for physiological functioning.
In particular, a different mechanism operates for glycolipids—the lipids that show the most striking and consistent asymmetric distribution in animal cells.
These help organize membrane components into localized areas that are involved in specific processes, such as signal transduction.
The bilayer of red blood cells is composed of cholesterol and phospholipids in equal proportions by weight.
In the bilayer, the sugar groups of glycolipids are exposed at the cell surface, where they can form hydrogen bonds.
[10] Glycolipids perform a vast number of functions in the biological membrane that are mainly communicative, including cell recognition and cell-cell adhesion.
This means that the size, charge, and other chemical properties of the atoms and molecules attempting to cross it will determine whether they succeed in doing so.
Generally, small hydrophobic molecules can readily cross phospholipid bilayers by simple diffusion.
Plasma membranes can also form different types of "supramembrane" structures such as caveolae, postsynaptic density, podosome, invadopodium, desmosome, hemidesmosome, focal adhesion, and cell junctions.
The hydrophobic core of the phospholipid bilayer is constantly in motion because of rotations around the bonds of lipid tails.
However, because of hydrogen bonding with water, the hydrophilic head groups exhibit less movement as their rotation and mobility are constrained.
[14] The transition temperature depends on such components of the lipid bilayer as the hydrocarbon chain length and the saturation of its fatty acids.
These organisms maintain a constant fluidity by modifying membrane lipid fatty acid composition in accordance with differing temperatures.
It enables membrane proteins to diffuse rapidly in the plane of the bilayer and to interact with one another, as is crucial, for example, in cell signaling.
It permits membrane lipids and proteins to diffuse from sites where they are inserted into the bilayer after their synthesis to other regions of the cell.
[5] The fluidity property is at the center of the Helfrich model which allows for calculating the energy cost of an elastic deformation to the membrane.