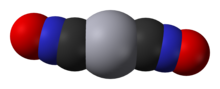Mercury(II) fulminate
It is highly sensitive to friction, heat and shock and is mainly used as a trigger for other explosives in percussion caps and detonators.
First used as a priming composition in small copper caps beginning in the 1820s, mercury fulminate quickly replaced flints as a means to ignite black powder charges in muzzle-loading firearms.
[1] Mercury fulminate has the distinct advantage over potassium chlorate of being non-corrosive, but it is known to weaken with time, by decomposing into its constituent elements.
The reduced mercury which results forms amalgams with cartridge brass, weakening it, as well.
In addition, none of these compounds requires mercury for manufacture, supplies of which can be unreliable in wartime.