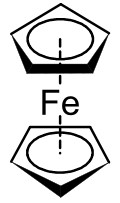
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (C5H−5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M.
Some metallocenes consist of metal plus two cyclooctatetraenide anions (C8H2−8, abbreviated cot2−), namely the lanthanocenes and the actinocenes (uranocene and others).
The first metallocene to be classified was ferrocene, and was discovered simultaneously in 1951 by Kealy and Pauson,[2] and Miller et al.[3] Kealy and Pauson were attempting to synthesize fulvalene through the oxidation of a cyclopentadienyl salt with anhydrous FeCl3 but obtained instead the substance C10H10Fe[2] At the same time, Miller et al reported the same iron product from a reaction of cyclopentadiene with iron in the presence of aluminum, potassium, or molybdenum oxides.
Using the nomenclature of "hapticity", the equivalent bonding of all 5 carbon atoms of a cyclopentadienyl ring is denoted as η5, pronounced "pentahapto".
In contrast to the more strict definition proposed by International Union of Pure and Applied Chemistry, which requires a d-block metal and a sandwich structure, the term metallocene and thus the denotation -ocene, is applied in the chemical literature also to non-transition metal compounds, such as barocene (Cp2Ba), or structures where the aromatic rings are not parallel, such as found in manganocene or titanocene dichloride (Cp2TiCl2).
It reacts with metal halides to give thallium chloride, which is poorly soluble, and the cyclopentadienyl complex.
For Group IV metallocenes, donor solvents like ether or THF are distinctly undesirable for polyolefin catalysis.
A structural trend for the series MCp2 involves the variation of the M-C bonds, which elongate as the valence electron count deviates from 18.
Infrared and Raman spectroscopies have proved to be important in the analysis of cyclic polyenyl metal sandwich species, with particular use in elucidating covalent or ionic M–ring bonds and distinguishing between central and coordinated rings.
Some typical spectral bands and assignments of iron group metallocenes are shown in the following table:[8] Nuclear magnetic resonance (NMR) is the most applied tool in the study of metal sandwich compounds and organometallic species, giving information on nuclear structures in solution, as liquids, gases, and in the solid state.
[8] After the discovery of ferrocene, the synthesis and characterization of derivatives of metallocene and other sandwich compounds attracted researchers’ interests.
The ferrocene/ferrocenium biosensor has been discussed for determining the levels of glucose in a sample electrochemically through a series of connected redox cycles.
[8] Metallocene dihalides [Cp2MX2] (M = Ti, Mo, Nb) exhibit anti-tumor properties, although none have proceeded far in clinical trials.