Metastasis
The cells which constitute the tumor eventually undergo metaplasia, followed by dysplasia then anaplasia, resulting in a malignant phenotype.
Evidence suggests that CTC clusters may retain their multicellular configuration throughout metastasis, enhancing their ability to establish secondary tumors.
[7] This perspective aligns with the cancer exodus hypothesis, which posits that maintaining this cluster structure contributes to a higher metastatic potential.
[11] Understanding the enigma of cancer cell spread to distant sites, which accounts for over 90% of cancer-related deaths, necessitates comprehensive investigation.
Key outstanding questions revolve around the survival and migration of cancer cells, such as the nucleus, as they face challenges in passage through capillary valves and hydrodynamic shear forces in the circulation system, making CTCs an unlikely source of metastasis.
Moreover, understanding how cancer cells adapt to the metastatic niche and remain dormant (tumor dormancy) for extended periods presents difficult questions that require further investigation.
[11] Metastasis involves a complex series of steps in which cancer cells leave the original tumor site and migrate to other parts of the body via the bloodstream, via the lymphatic system, or by direct extension.
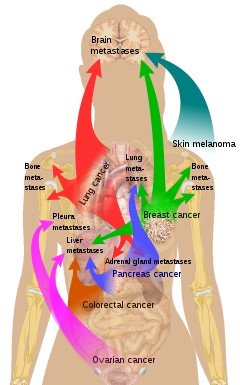
The location of the metastases is not always random, with different types of cancer tending to spread to particular organs and tissues at a rate that is higher than expected by statistical chance alone.
[20][21] Endothelial progenitor cells are important in tumor growth, angiogenesis and metastasis, and can be marked using the Inhibitor of DNA Binding 1 (ID1).
This novel finding meant that investigators gained the ability to track endothelial progenitor cells from the bone marrow to the blood to the tumor-stroma and even incorporated in tumor vasculature.
Furthermore, ablation of the endothelial progenitor cells in the bone marrow can lead to a significant decrease in tumor growth and vasculature development.
[22] The immune system is typically deregulated in cancer and affects many stages of tumor progression, including metastasis.
[citation needed] Epigenetic regulation also plays an important role in the metastatic outgrowth of disseminated tumor cells.
[24] A recent study shows that PKC-iota promotes melanoma cell invasion by activating Vimentin during EMT.
These results suggested that PKC-ι is involved in signaling pathways which upregulate EMT in melanoma thereby directly stimulates metastasis.
[25] Recently, a series of high-profile experiments suggests that the co-option of intercellular cross-talk mediated by exosome vesicles is a critical factor involved in all steps of the invasion-metastasis cascade.
Localized spread to regional lymph nodes near the primary tumor is not normally counted as a metastasis, although this is a sign of a worse outcome.
Because of their thinner walls, veins are more frequently invaded than are arteries, and metastasis tends to follow the pattern of venous flow.
[citation needed] According to the seed and soil theory, it is difficult for cancer cells to survive outside their region of origin, so in order to metastasize they must find a location with similar characteristics.
Malignant melanoma spreads to the brain, presumably because neural tissue and melanocytes arise from the same cell line in the embryo.
[30] In 1928, James Ewing challenged the seed and soil theory, and proposed that metastasis occurs purely by anatomic and mechanical routes.
This hypothesis has been recently utilized to suggest several hypotheses about the life cycle of circulating tumor cells (CTCs) and to postulate that the patterns of spread could be better understood through a 'filter and flow' perspective.
[31] However, contemporary evidences indicate that the primary tumour may dictate organotropic metastases by inducing the formation of pre-metastatic niches at distant sites, where incoming metastatic cells may engraft and colonise.
[33] Studies have shown that, if simple questioning does not reveal the cancer's source (coughing up blood—"probably lung", urinating blood—"probably bladder"), complex imaging will not either.
Still, the determination of the primary tumor can often be very difficult, and the pathologist may have to use several adjuvant techniques, such as immunohistochemistry, FISH (fluorescent in situ hybridization), and others.
[35] The identification of this metastasis-associated signature provides promise for identifying cells with metastatic potential within the primary tumor and hope for improving the prognosis of these metastatic-associated cancers.
[35] Treatment and survival is determined, to a great extent, by whether or not a cancer remains localized or spreads to other locations in the body.
[42] In another experiment they found that elasticity of melanoma cells is important for its metastasis and growth: non-pigmented tumors were bigger than pigmented and it was much easier for them to spread.
[42] The first physician to report the possibility of local metastasis from a primary cancerous source to nearby tissues was Ibn Sina.
[45][46][47] Metastasis is an Ancient Greek word (μετάστασις) meaning "displacement", from μετά, meta, "next", and στάσις, stasis, "placement".