Methane clathrate
[1][2][3][4][5][6] Originally thought to occur only in the outer regions of the Solar System, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediments on the ocean floors of the Earth (around 1100 m below the sea level).
Methane clathrates are common constituents of the shallow marine geosphere and they occur in deep sedimentary structures and form outcrops on the ocean floor.
[8] The ice-core methane clathrate record is a primary source of data for global warming research, along with oxygen and carbon dioxide.
In this scenario, heating causes catastrophic melting and breakdown of primarily undersea hydrates, leading to a massive release of methane and accelerating warming.
[11] As the result, methane hydrates are no longer considered one of the tipping points in the climate system, and according to the IPCC Sixth Assessment Report, no "detectable" impact on the global temperatures will occur in this century through this mechanism.
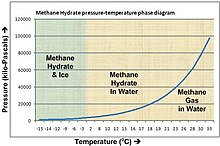
The observed density is around 0.9 g/cm3, which means that methane hydrate will float to the surface of the sea or of a lake unless it is bound in place by being formed in or anchored to sediment.
[25][26] In the less common second type found near the sediment surface, some samples have a higher proportion of longer-chain hydrocarbons (< 99% methane) contained in a structure II clathrate.
Carbon from this type of clathrate is isotopically heavier (δ13C is −29 to −57 ‰) and is thought to have migrated upwards from deep sediments, where methane was formed by thermal decomposition of organic matter.
Below this region of aerobic activity, anaerobic processes take over, including, successively with depth, the microbial reduction of nitrite/nitrate, metal oxides, and then sulfates are reduced to sulfides.
This production of methane is a rather complicated process, requiring a highly reducing environment (Eh −350 to −450 mV) and a pH between 6 and 8, as well as a complex syntrophic, consortia of different varieties of archaea and bacteria.
These modern estimates are notably smaller than the 10,000 to 11,000 Gt C (2×1016 m3) proposed[40] by previous researchers as a reason to consider clathrates to be a geo-organic fuel resource (MacDonald 1990, Kvenvolden 1998).
Methane clathrates in continental rocks are trapped in beds of sandstone or siltstone at depths of less than 800 m. Sampling indicates they are formed from a mix of thermally and microbially derived gas from which the heavier hydrocarbons were later selectively removed.
[36] Other problems facing commercial exploitation are detection of viable reserves and development of the technology for extracting methane gas from the hydrate deposits.
[36] Bjørn Kvamme and Arne Graue at the Institute for Physics and technology at the University of Bergen have developed a method for injecting CO2 into hydrates and reversing the process; thereby extracting CH4 by direct exchange.
[46] The University of Bergen's method is being field tested by ConocoPhillips and state-owned Japan Oil, Gas and Metals National Corporation (JOGMEC), and partially funded by the U.S. Department of Energy.
[49] The hydrate field from which the gas was extracted is located 50 kilometres (31 mi) from central Japan in the Nankai Trough, 300 metres (980 ft) under the sea.
"[49] Japan estimates that there are at least 1.1 trillion cubic meters of methane trapped in the Nankai Trough, enough to meet the country's needs for more than ten years.
This is commonly achieved by removing water, or by the addition of ethylene glycol (MEG) or methanol, which act to depress the temperature at which hydrates will form.
[66] However, the East Siberian Arctic Shelf averages 45 meters in depth, and it is assumed that below the seafloor, sealed by sub-sea permafrost layers, hydrates deposits are located.
[67][68] This would mean that when the warming potentially talik or pingo-like features within the shelf, they would also serve as gas migration pathways for the formerly frozen methane, and a lot of attention has been paid to that possibility.
A release on this scale would increase the methane content of the planet's atmosphere by a factor of twelve,[72][73] equivalent in greenhouse effect to a doubling in the 2008 level of CO2.
This is what led to the original Clathrate gun hypothesis, and in 2008 the United States Department of Energy National Laboratory system[74] and the United States Geological Survey's Climate Change Science Program both identified potential clathrate destabilization in the Arctic as one of four most serious scenarios for abrupt climate change, which have been singled out for priority research.
At the time, some of the melting was thought to be the result of geological heating, but more thawing was believed to be due to the greatly increased volumes of meltwater being discharged from the Siberian rivers flowing north.
[84] By 2013, the same team of researchers used multiple sonar observations to quantify the density of bubbles emanating from subsea permafrost into the ocean (a process called ebullition), and found that 100–630 mg methane per square meter is emitted daily along the East Siberian Arctic Shelf (ESAS), into the water column.
However, Arctic cyclones, fueled by global warming, and further accumulation of greenhouse gases in the atmosphere could contribute to more rapid methane release from this source.
They conclude that the increased methane flux started hundreds to thousands of years ago, noted about it, "..episodic ventilation of deep reservoirs rather than warming-induced gas hydrate dissociation.
Understanding how methane interacts with other important geological, chemical and biological processes in the Earth system is essential and should be the emphasis of our scientific community.
[88]Research by Wallmann et al. (2018) concluded that hydrate dissociation at Svalbard 8,000 years ago was due to isostatic rebound (continental uplift following deglaciation).
[89] Moreover, another paper published in 2017 found that only 0.07% of the methane released from the gas hydrate dissociation at Svalbard appears to reach the atmosphere, and usually only when the wind speeds were low.
[98][99] With the inclusion of tetrahydrofuran, though there is a slight reduction in the gas storage capacity, the hydrates have been demonstrated to be stable for several months in a recent study at −2 °C and atmospheric pressure.

Inset: clathrate structure (University of Göttingen, GZG. Abt. Kristallographie).
Source: United States Geological Survey .

Source: USGS







