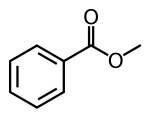
Methyl benzoate
It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents.
Methyl benzoate has a pleasant smell, strongly reminiscent of the fruit of the feijoa tree, and it is used in perfumery.
Electrophiles attack the ring, illustrated by acid-catalysed nitration with nitric acid to give methyl 3-nitrobenzoate.
Nucleophiles attack the carbonyl center, illustrated by hydrolysis with addition of aqueous NaOH to give methanol and sodium benzoate.
[3] It is one of many compounds that is attractive to males of various species of orchid bees, which apparently gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.